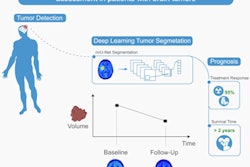
Using PET with the radiolabeled amino acid F-18 fluoroethyl tyrosine (F-18 FET) can significantly help determine the efficacy of radiotherapy in certain cancer patients who develop brain metastases, according to a study published online September 4 in the Journal of Nuclear Medicine.
German and Swiss researchers achieved an accuracy of 85% when using FET-PET and easily calculated tumor-to-brain ratios to distinguish relapses of brain metastases from changes related to treatments that included immune checkpoint inhibition or targeted therapy. By doing so, F-18 FET-PET also bested the performance of contrast-enhanced MRI.
"F-18 FET-PET provided additional important clinical information for treatment response evaluation beyond the information provided by contrast-enhanced MRI alone, i.e., in terms of identification of false-positive or false-negative MRI findings," added the authors, led by Dr. Norbert Galldiks from University Hospital Cologne.
Between 20% and 40% of patients with late-stage cancer develop brain metastases, which naturally further jeopardize their prognosis. To improve chances for survival, oncologists increasingly have turned to immunotherapy with immune checkpoint inhibition and targeted therapy, particularly for patients with melanoma or lung cancer.
While contrast-enhanced MRI has been used to determine the efficacy of immunotherapy, the results "can be highly variable ... and the interpretation regarding the differentiation of treatment-related changes from brain metastases relapse is often difficult," Galldiks and colleagues cautioned.
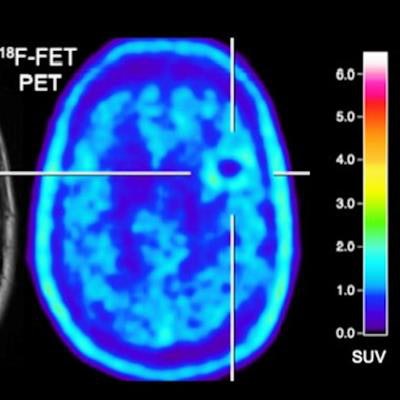
Conversely, there is evidence -- albeit limited -- that PET imaging with radiolabeled amino acids might advance treatment evaluation. Amino acid uptake, as with FET, increases in neoplastic tissue, which could indicate cancer cell growth, and it has low uptake in normally functioning brain tissue. As a result, there is enhanced tumor-to-brain contrast for better interpretation, the authors noted.
To test the benefits of FET-PET, Galldiks et al performed a retrospective study that included 40 patients (mean age, 59 ±13 years; range, 27-83 years) diagnosed between 2015 and 2019 with a total of 107 metastatic brain tumors related to either malignant melanoma or non-small cell lung cancer. All but three patients received radiotherapy to treat their cancer.
Prior to F-18 FET-PET, 28 patients (70%) underwent radiotherapy, either as single modality or in combination with immune checkpoint inhibition or targeted therapy. There was a total of 60 F-18 FET-PET/CT scans for the 40 subjects, who also underwent gadolinium-enhanced 1.5- or 3-tesla MRI, to determine the success of their treatment.
Using a tumor-to-background ratio of 1.95 as a threshold to differentiate brain metastases from treatment-related changes, the researchers could significantly distinguish relapse of brain metastases from treatment changes with an accuracy of 85% (p = 0.003) with FET-PET. The modality also identified patients who were both responders and nonresponders to treatment that included immune checkpoint inhibition or targeted therapy with accuracy, sensitivity, and specificity between 80% and 83%.
In addition, FET-PET showed patients who responded to immune checkpoint inhibition or targeted therapy achieved a significantly longer stable follow-up (median time, 10.5 months), compared with those who did not respond to treatment (median time, four months) (p = 0.004).
In comparison with MRI, FET-PET provided additional information in four (31%) of 13 patients who showed treatment-related changes beyond contrast-enhanced MRI alone. For example, FET-PET confirmed a "significant decrease of metabolic activity" in two of the four cases, whereas MRI indicated metastatic disease progression in both patients.
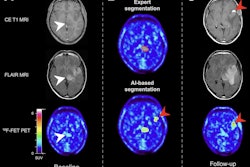
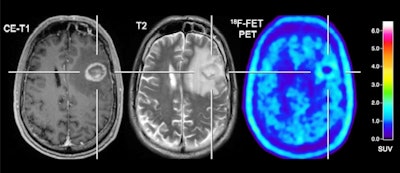 Patient with a melanoma brain metastasis pretreated with radiosurgery concurrent with nivolumab and dabrafenib in combination with trametinib. In contrast to the progressive MRI, amino acid F-18 FET-PET showed no significant uptake and was consistent with treatment-related changes. After PET imaging, the survival time was 24 months. Images courtesy of the Journal of Nuclear Medicine.
Patient with a melanoma brain metastasis pretreated with radiosurgery concurrent with nivolumab and dabrafenib in combination with trametinib. In contrast to the progressive MRI, amino acid F-18 FET-PET showed no significant uptake and was consistent with treatment-related changes. After PET imaging, the survival time was 24 months. Images courtesy of the Journal of Nuclear Medicine."The detection of brain metastases relapse with high accuracy as well as the reliable assessment of response is essential for optimizing patient counseling as well as the treatment concept for each individual patient," Galldiks and colleagues concluded. "Furthermore, this approach achieves an accuracy that is sufficient to influence clinical decision-making and may therefore help to reduce the number of invasive diagnostic interventions and overtreatment for a considerable number of seriously ill patients with brain metastases."