PET imaging can identify whether or not patients respond to an investigational treatment for prostate cancer, according to a pilot study published in the September issue of the Journal of Nuclear Medicine.
Johns Hopkins University clinicians analyzed PET/CT imaging with the radiotracer F-18 DCFPyL in patients before and after they began bipolar androgen therapy (BAT) for metastatic castration-resistant prostate cancer (mCRPC).
"New radiotracer-avid lesions on F-18 DCFPyL-PET/CT in men with mCRPC undergoing BAT can indicate early progression," wrote oncologist Dr. Mark Markowski, PhD, and colleagues.
The finding may fill a need for a new imaging strategy to determine whether patients will respond to bipolar androgen therapy, according to the authors, who pioneered the strategy.
Androgen (testosterone) deprivation therapy is the standard treatment approach for men with prostate cancer, but often the treatment stops working because prostate cancer cells adapt to the lower levels. Paradoxically, however, research has also shown that high concentrations of androgens are able to suppress tumor growth.
In this study, the investigators aimed to determine if changes in F-18 DCFPyL-PET/CT imaging could detect changes in patients after treatment with BAT in men with mCRPC.
The researchers enlisted six men with mCRPC. Patients receiving bipolar androgen therapy were imaged with F-18 DCFPyL PET/CT at baseline and after three months of treatment. Progression by PSMA-targeted PET/CT was defined as the appearance of any new lesion that was avid for the F-18 DCFPyL tracer. All participants had mCRPC and prior treatment with at least one novel androgen receptor-targeted therapy.
PET/CT images were acquired on either a Biograph mCT 128-slice scanner (Siemens Healthineers) or a Discovery RX 64-slice scanner (GE Healthcare) using 3D emission mode with CT-based attenuation correction.
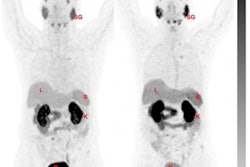
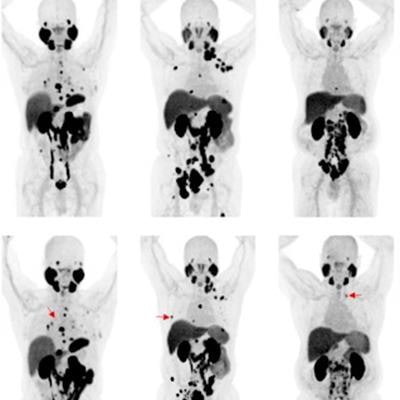
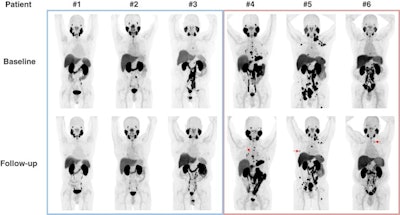 Changes in F-18 DCFPyL-PET/CT imaging after three months of bipolar androgen therapy. Baseline and follow-up maximum intensity projection whole-body images are shown for each patient. For patients 4-6, representative new lesions or sites of progression are indicated by red arrows. Image courtesy of the Journal of Nuclear Medicine.
Changes in F-18 DCFPyL-PET/CT imaging after three months of bipolar androgen therapy. Baseline and follow-up maximum intensity projection whole-body images are shown for each patient. For patients 4-6, representative new lesions or sites of progression are indicated by red arrows. Image courtesy of the Journal of Nuclear Medicine.Three of six (50%) patients had progression on F-18 DCFPyL PET/CT. All three had stable disease or better on contemporaneous conventional imaging. Radiographic progression on CT or bone scanning was observed within three months of progression on F-18 DCFPyL PET/CT.
In addition, for the three patients who did not have progression on F-18 DCFPyL-PET/CT, radiographic progression was not observed for at least six months.
"New radiotracer-avid lesions on F-18 DCFPyL-PET/CT in men with mCRPC undergoing BAT can indicate early progression," the researchers found.
The study was limited by the small number of patients and the use of only two imaging time points, the authors wrote. In addition, the fact that all scans were read by a single radiologist (although masked) could bias the results of the study.
Nonetheless, the findings warrant further research, Markowski and colleagues concluded.
"A larger prospective study is underway to confirm these findings," they wrote.