Molecular imaging developer Sofie Biosciences has submitted an investigational new drug application (IND) with the U.S. Food and Drug Administration (FDA) for a radiopharmaceutical targeting the fibroblast activation protein inhibitor (FAPI).
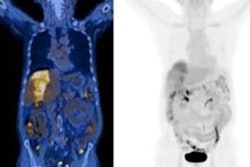
The IND is for a phase II, multicenter, single-blind, nonrandomized study of gallium-68 (Ga-68) FAPI-46 PET imaging in patients with pancreatic ductal adenocarcinoma, according to the vendor.
The filing enables select institutions to participate with Sofie in generating clinically relevant diagnostic imaging data to support ongoing work in pancreatic imaging, as well as efforts for other solid tumors and nononcologic applications, the company said.