The U.S. Centers for Medicare and Medicaid Services (CMS) has proposed a national coverage determination (NCD) that would limit coverage for drugs like aducanumab only to people enrolled in qualifying clinical trials.
The proposal would apply to approved monoclonal antibodies that target beta-amyloid plaque for the treatment of Alzheimer's disease. Currently, aducanumab (Aduhelm, Biogen) is the only such monoclonal antibody approved by the U.S. Food and Drug Administration (FDA). The NCD proposal is open to public comment for 30 days.
If the proposed NCD is finalized, CMS will review each submitted clinical trial to determine whether it meets specific criteria. For example, the diversity of patients in each trial must be representative of the national population diagnosed with Alzheimer's disease.
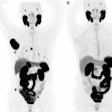
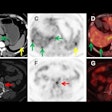
In addition, for any approved trial that includes a beta-amyloid PET scan as part of the protocol, CMS will cover one beta-amyloid PET scan per patient if the patient did not previously receive a beta-amyloid PET scan, according to the draft.
"This proposed National Coverage Determination is the result of robust evidence analysis conducted through a thorough review process that found while there may be the potential for promise with [Aduhelm], there is also the potential for harm to patients," said CMS Administrator Chiquita Brooks-LaSure in a press release.
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) reacted with concern to the CMS proposal limiting patients to one PET scan, given that beta-amyloid PET is FDA-approved for use in Alzheimer's disease.
"We are concerned that CMS did not significantly change their [coverage with evidence development (CED)] requirement for beta-amyloid PET scans," the SNMMI said in a statement.
CMS’s decision to cover Alzheimer’s therapy under CED is an incomplete solution rather than a productive resolution that would allow widespread and equitable patient access to monoclonal antibody therapy or clinical trials of that treatment, the organization said.
Aduhelm was approved using the FDA's accelerated approval pathway, which can be used for a drug for a serious or life-threatening illness that provides a meaningful therapeutic advantage over existing treatments. Experts who included members of the FDA advisory board that reviewed aducanumab prior to approval criticized the process, citing a lack of evidence on the drug's effectiveness.
After reviewing all comments received on the proposed determination, CMS will announce its final decision by April 11, 2022. The public can submit comments on the CMS.gov website.