Spinal fluid and blood tests may help recruit patients for PET studies of new Alzheimer's disease treatments, according to research published February 28 in Alzheimer's & Dementia: The Journal of the Alzheimer's Association.
A Swiss group led by senior author Michael Schöll, PhD, of the University Gothenburg investigated whether fluid and blood biomarkers that indicate Alzheimer's disease could predict the accumulation of neurofibrillary tau tangles seen on PET scans in patients diagnosed with the disease. They found the lab tests performed well enough to help select patients for PET imaging studies evaluating new treatments.
"The identification of accessible and cost-effective biomarkers that can predict longitudinal accumulation of aggregated tau as measured by tau PET is key to facilitate the search for tau-targeting treatments," wrote Schöll and colleagues.
A widely held view on the development of Alzheimer's disease suggests that beta-amyloid deposits in the brain initiate the spread of misfolded tau protein and that the accumulation of misfolded tau protein (tau "tangles") in turn causes neurodegeneration.
Thus, clinical trials of new therapies that target tau in Alzheimer's disease need to recruit individuals at risk of tau accumulation.
To date, beta-amyloid PET scans are the only established Alzheimer's disease biomarker that has consistently been found to predict longitudinal tau accumulation in cognitively unimpaired patients. Yet associated costs and limited accessibility hamper its use in recruiting patients for clinical trials, according to the authors.
Core cerebrospinal fluid biomarkers for Alzheimer's disease are more affordable and accessible than beta-amyloid PET, and they could allow the simultaneous assessment of both beta amyloid and soluble tau pathology using a single procedure, the group wrote.
In this study, the researchers investigated how cerebral spinal fluid Alzheimer's disease biomarkers and plasma phosphorylated tau-181 (p-tau181) associate with longitudinal tau accumulation, as measured by tau PET, and compared their predictive performance head-to-head with beta-amyloid PET.
Further, Scholl and colleagues looked at potential implications on the use of fluid biomarkers for clinical trial designs that use longitudinal tau PET as an outcome measure.
The researchers identified older individuals who participated in earlier Alzheimer's Disease Neuroimaging Initiative studies and had serial tau PET scans (florbetapir or florbetaben), baseline beta-amyloid PET scans, and baseline fluid biomarker (n = 163) or plasma p-tau181 (n = 74) tests. Patients were cognitively unimpaired (CU) or had mild cognitive impairment (MCI) or dementia (CI).
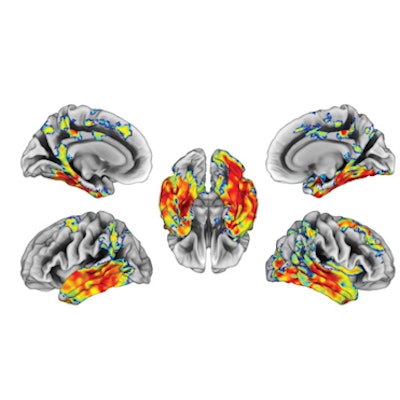
A voxel-wise analysis revealed that baseline levels of all cerebrospinal fluid biomarkers showed moderate to strong correlations with longitudinal F-18 flortaucipir standardized uptake value ratio (SUVR) changes among CU and MCI patients. Plasma p-tau181 was more closely correlated with F-18 flortaucipir SUVR change in patients with dementia, and it predicted tau accumulation with similar but slightly higher values than beta-amyloid PET.
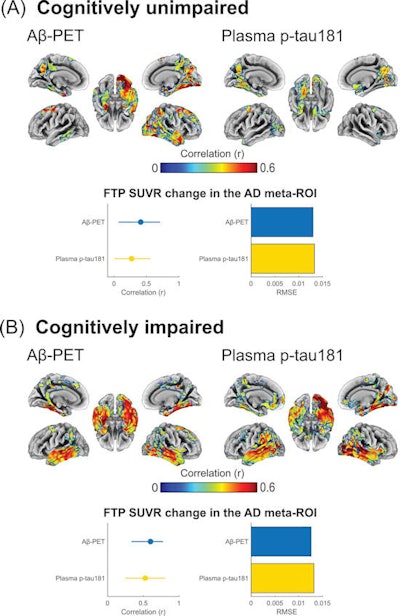
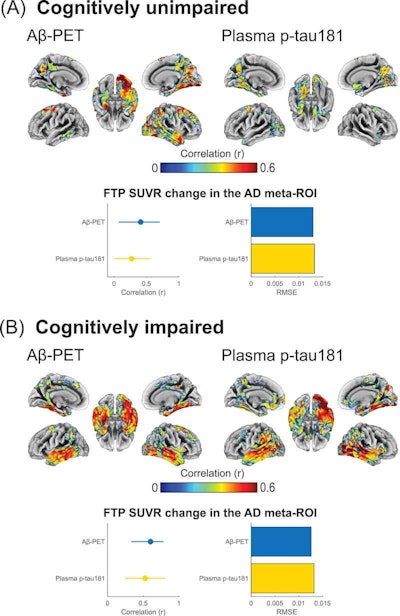
Associations of baseline beta-amyloid PET and baseline phosphorylated tau-181 (p-tau181) with F-18 flortaucipir (FTP) Standardized uptake value ratio (SUVR) annualized change for (A) cognitively unimpaired and (B) cognitively impaired individuals. Brain surface renderings depict Pearson correlation coefficients adjusted for age and sex (r) representing the strength of the association between the different biomarkers and F-18 flortaucipir SUVR change in each brain region. The right upper panel represents age- and sex-adjusted Pearson correlation coefficients (r), along with p-values, for the association between the biomarkers and F-18 flortaucipir SUVR change in the Alzheimer's disease meta-region of interest (ROI). The root mean squared error (RMSE) for the prediction of F-18 flortaucipir SUVR change by a linear model with the biomarker as predictor and age and sex as covariates. RMSE was estimated using leave-one-out cross-validation. Voxel-wise statistical maps were thresholded using more lenient cluster-forming thresholds of p < 0.05 (uncorrected) at the voxel level and further thresholded at the cluster level by restricting results to clusters with a number of voxels higher than the expected number of voxels as predicted using random field theory. Image courtesy of Alzheimer's & Dementia: The Journal of the Alzheimer's Association.
Cerebrospinal fluid and p-tau181 levels were significantly associated with F-18 flortaucipir SUVR change in patients who were positive for beta-amyloid plaque, but negative for tau accumulation, according to the findings. Higher plasma p-tau-181 levels were also associated with faster F-18 flortaucipir SUVR change rates among beta-amyloid-positive yet tau-negative participants, suggesting that elevations of these markers may precede early tau aggregation, the authors wrote.
Finally, the group investigated hypothetical implications of the use of fluid biomarkers in clinical trials that use tau PET endpoints and found participant enrollment based on fluid biomarkers could result in comparable required sample sizes for trial inclusion based on beta-amyloid PET, with similar screening failure rates.
"Our study suggests that [cerebrospinal fluid] biomarkers and plasma p-tau181 represent cost-effective predictors of longitudinal tau accumulation as measured by tau PET, being capable to anticipate future tau aggregation even at very early stages of tau deposition," the group wrote.
The group noted limitations, namely that the number of beta-amyloid-positive yet tau-negative subjects and the number of subjects who progressed to tau-positive at follow-up was limited. For this reason, the analyses involving these participants should be considered exploratory. Also, the limited number of participants did not allow for an accurate evaluation of plasma p-tau181 as a standalone test for participant selection in trials with tau PET outcomes.
Nonetheless, the use of fluid biomarkers may be an effective strategy for participant inclusion in anti-tau clinical trials, as it resulted in a similar enrichment as for beta-amyloid PET, the researchers wrote.
"Future comparative studies using larger sample sizes are warranted to evaluate the value of plasma p-tau181 and [cerebrospinal fluid] as standalone biomarkers for sample enrichment," Schöll and colleagues concluded.