What's the best way to pick which patients should receive treatment with Pluvicto, a recently approved radiopharmaceutical therapy for metastatic prostate cancer? Imaging criteria can help select the best candidates, according to a presentation May 2 at the American Roentgen Ray Society (ARRS) meeting in New Orleans.
Dr. Phillip Kuo, PhD, of the University of Arizona in Tucson presented research at ARRS 2022 on the novel imaging criteria their group used in trials of Pluvicto, a lutetium-177 (Lu-177) prostate-specific membrane antigen 617 (PSMA-617) radioligand therapy intended for patients with metastatic prostate cancer.
The criteria can provide a guide moving forward for use of the drug in the real world, Kuo said.
"This is a classic example when you're trying to develop an imaging criteria for a clinical trial, you also want to make it so that it will eventually be applicable to real-life if the drug ever gets approved," he said.
The U.S. Food and Drug Administration (FDA) approved Pluvicto from Novartis in March 2022 for adult patients with PSMA-positive metastatic castration-resistant prostate cancer who have previously received other anticancer therapies, such as androgen receptor therapy or taxane-based chemotherapy.
In addition, the FDA approved a kit from Novartis (Locametz) for the preparation of the radiotracer gallium-68 (Ga-68) PSMA, which is used in PET imaging to identify metastatic prostate cancer tumors.
More than 248,000 cases of prostate cancer and approximately 34,000 deaths are projected in the U.S. in 2022. With approximately 40% of patients seeing cancer return after treatment, an estimated 40,000 patients per year may be candidates for Pluvicto treatment, Kuo said.
First, new definitions for "PSMA-positive" and "PSMA-negative" were created specifically for the VISION trial. The definition of "PSMA-positivity" was designed not to localize prostate cancer, but to identify tumors with sufficient target expression that would likely respond to therapy.
"What we consider a PSMA-positive lesion is not whether or not we think it is prostate cancer, but whether it has activity greater than liver," Kuo said.
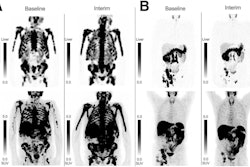
That is, if Ga-68 PSMA-PET/CT scans reveal one or more lesions in any organ and of any size with uptake activity greater than the liver on visual inspection, the patient would be a candidate for therapy, Kuo said.
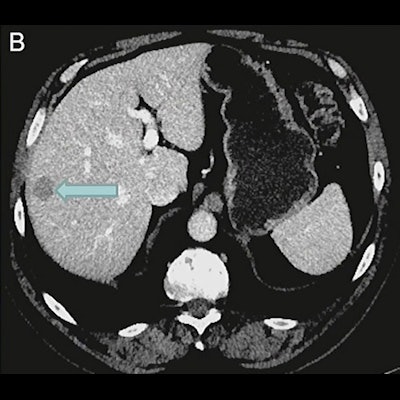
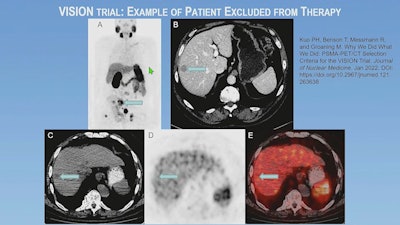 An example of a patient excluded by imaging selection criteria used in the VISION trial. (A) Anterior maximum-intensity projection from Ga-68 PSMA-11 PET/CT has readily evident PSMA-positive disease, for example, in the sacrum (arrow); thus, the next step is to evaluate the diagnostic CT scan for lesions that fulfill size criteria for possible exclusion. (B) On contrast-enhanced diagnostic CT, a 1.8-cm metastasis is identified in the right hepatic lobe (arrow). Trans-axial low-dose CT (C), PET (D), and fused PET/CT (E) images show that the metastasis (arrows) has activity similar to normal liver, is therefore PSMA-negative, and would result in exclusion. Level of uptake in the hepatic metastasis was importantly confirmed in sagittal and coronal planes (not shown) in case of misregistration.
An example of a patient excluded by imaging selection criteria used in the VISION trial. (A) Anterior maximum-intensity projection from Ga-68 PSMA-11 PET/CT has readily evident PSMA-positive disease, for example, in the sacrum (arrow); thus, the next step is to evaluate the diagnostic CT scan for lesions that fulfill size criteria for possible exclusion. (B) On contrast-enhanced diagnostic CT, a 1.8-cm metastasis is identified in the right hepatic lobe (arrow). Trans-axial low-dose CT (C), PET (D), and fused PET/CT (E) images show that the metastasis (arrows) has activity similar to normal liver, is therefore PSMA-negative, and would result in exclusion. Level of uptake in the hepatic metastasis was importantly confirmed in sagittal and coronal planes (not shown) in case of misregistration.The use of a cut-off point based on standardized uptake value (SUV) was not chosen for the VISION trial because of the variability of SUV across clinical sites and known challenges of standardization, he added.
The next step involved reviewing CT and MRI scans to identify lesions based on size, Kuo said.
Criteria for assessing anatomic imaging were divided into three systems -- lymph nodes, solid organs, and the skeleton -- with the following criteria:
- Lymph nodes were assessed if lesions were 2.5 cm or larger in the short axis.
- For solid parenchymal metastases, only disease 1.0 cm in the short axis or greater were assessed for PSMA-status.
- Bone metastases with a soft-tissue component 1.0 cm in the short axis or greater, and only the soft-tissue component, were assessed.
"If you've met one of these size criteria on CT, then you need to assess those lesions that met the type size criteria, and if even one of those lesions was PSMA-negative, which means uptake the same or less than liver, you're excluded from therapy," Kuo explained.
Ultimately, about 15% of patients were excluded from the randomization arm of the VISION trial due to the imaging criteria.
As for the effectiveness of the drug itself, Lu-177 PSMA-617 improved overall survival for patients from 11.3 months to 15.3 months versus the standard of care.
"Four months may not sound like a lot, but in this group of patients who are rapidly progressing and have poor survival, this is a good improvement," Kuo concluded.