PET imaging with a recently approved prostate-specific membrane antigen (PSMA) radiotracer could improve cancer treatment planning for a large number of men with high-risk disease, according to a study published May 24 in Radiology.
Canadian researchers compared the performance of PET imaging with the radiotracer F-18 DCFPyL (Pylarify, Lantheus Medical Imaging) to conventional imaging in men with high-risk prostate cancer. For treatment planning, Pylarify helped detect more cancer, and it resulted in treatment changes in 22% of men.
"Initial staging with F-18 DCFPyL PET helped to detect more nodal metastases and distant metastases than did CT, bone scan, and optional multiparametric MRI," wrote corresponding author Dr. Ur Metser, head of molecular imaging at the University of Toronto's Princess Margaret Hospital.
Pylarify was approved in the U.S. last year for prostate cancer imaging, with clinical trials showing the tracer is effective for identifying lesions in men with high-risk disease. However, those trials did not compare the approach with conventional imaging, and data on its use in primary staging for treatment are limited, the authors wrote.
In this clinical trial, Metser and colleagues hypothesized that F-18 DCFPyL PET would improve detection rates in men with metastatic disease, which would change treatment plans for a significant number of patients.
The researchers enrolled 108 men (median age, 66) with unfavorable intermediate- or high-risk prostate cancer between May 2018 and December 2020. Within 12 weeks of enrolling, patients underwent conventional imaging by contrast-enhanced CT, technetium-99m (Tc-99m) bone scintigraphy, and optional multiparametric prostate MRI.
Patients then underwent initial staging with F-18 DCFPyL PET/CT, or PET/MRI, and the researchers compared the intended treatment plan for patients before PET with the treatment plan established after performing PET.
Out of the 108 cases, F-18 DCFPyL PET outperformed conventional imaging by upstaging disease extent in 36 men (33%) and downstaging in seven men (7%). PET with the radiotracer also identified twice as many cases of oligometastatic disease, according to the findings.
PET altered treatment in 24 of 108 (22%) men, with the most frequent treatment change from systemic to local-regional therapy in 10 men, and from local-regional to systemic therapy in nine. Equivocal findings were encountered with PET in one of 108 men, compared with 29 of 108 with conventional imaging (27%).
"Initial staging with (F-18 DCFPyL) PET after conventional imaging (bone scan and CT with or without multiparametric MRI) helped to detect more nodal and distant metastases than conventional imaging alone," the authors wrote.
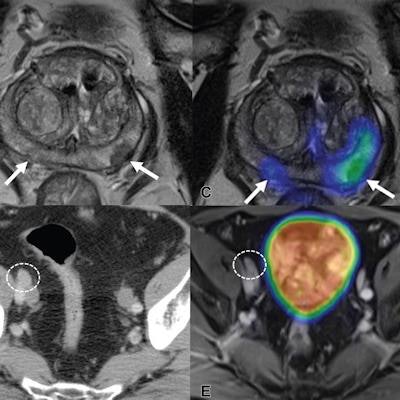
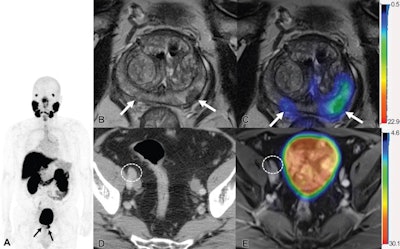 Images show F-18 DCFPyL PET/MRI and contrast-enhanced CT in a 65-year-old man with clinical T3 stage prostate cancer who underwent bone scintigraphy with negative findings (not shown). (A) Anteroposterior maximum intensity projection image of F-18 DCFPyL PET/MRI shows PSMA-avid primary tumor (arrows). (B) Axial T2-weighted MRI of the prostate and (C) corresponding fused F-18 DCFPyL PET/MRI in the prostate show multifocal PSMA-avid primary tumors involving bilateral peripheral zone of the gland (arrows). (D) Axial CT image shows a 1.1-cm right external iliac lymph node, suspicious for nodal metastasis, by size criteria (N1; circle). (E) Axial fused F-18 DCFPyL PET/MRI does not show corresponding increased tracer activity (N0; circle). At time of radical prostatectomy, lymph node sampling was negative for metastases (pT3a pN0). Image courtesy of Radiology.
Images show F-18 DCFPyL PET/MRI and contrast-enhanced CT in a 65-year-old man with clinical T3 stage prostate cancer who underwent bone scintigraphy with negative findings (not shown). (A) Anteroposterior maximum intensity projection image of F-18 DCFPyL PET/MRI shows PSMA-avid primary tumor (arrows). (B) Axial T2-weighted MRI of the prostate and (C) corresponding fused F-18 DCFPyL PET/MRI in the prostate show multifocal PSMA-avid primary tumors involving bilateral peripheral zone of the gland (arrows). (D) Axial CT image shows a 1.1-cm right external iliac lymph node, suspicious for nodal metastasis, by size criteria (N1; circle). (E) Axial fused F-18 DCFPyL PET/MRI does not show corresponding increased tracer activity (N0; circle). At time of radical prostatectomy, lymph node sampling was negative for metastases (pT3a pN0). Image courtesy of Radiology.The authors noted that treatment change after PET in this study was similar to that seen in a multicenter randomized trial of gallium-68 (Ga-68) PSMA-11 PET/CT in prostate cancer patients, compared with conventional imaging. Ga-68 PSMA-11 received U.S. approval for imaging in prostate cancer patients in December 2020, and it was the first PSMA tracer to achieve the mark.
Limitations of the study included that two different modalities (PET/CT and PET/MRI) were used, the authors wrote. They concluded that data from randomized trials are needed to determine whether the use of F-18 DCFPyL PET can translate to better clinical outcomes for patients.
In an accompanying editorial, Dr. Hossein Jadvar, PhD, a professor of radiology, urology, and biomedical engineering at University of Southern California, wrote that the study adds to strong evidence for a competitive advantage of PSMA-PET over conventional imaging.
"It is time to switch from conventional imaging to PSMA-PET in routine clinical practice," he wrote.
However, Jadvar noted that it is understood that it will take some time for "this disruptive imaging modality" to be recognized as the standard imaging in prostate cancer at all levels of the health care system, including patients, providers, professional associations, insurers, pharma, and government.
"Hopefully, this will occur sooner rather than later to obtain the best care for patients," he concluded.