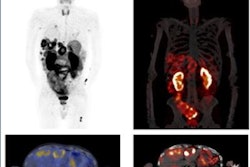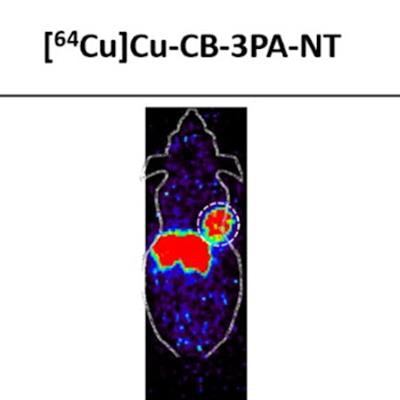
A new radionuclide-based agent offers potential as a theranostics approach for targeting multiple cancers, suggest findings presented at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
In his talk, Xinrui Ma from the University of North Carolina in Chapel Hill presented his team's findings, which showed that the agent successfully targets neurotensin receptors that are prevalent in a variety of cancers.
"The success of this theranostic approach has the potential to provide an accurate imaging-based method to efficiently detect neurotensin receptor expression in multiple types of cancer for diagnosis, patient screening, and treatment monitoring, as well as a radionuclide-based agent for therapy." Ma said. "This will ultimately lead to more personalized medicine for cancer patients."
Neurotensin receptors are overexpressed in several cancer types. These include lung, colorectal, breast, pancreatic, and prostate cancers. Previous research investigating the effects of synthesizing radiometal-labeled agents has shown moderate tumor uptake and retention.
Ma and colleagues wanted to test a series of neurotensin receptor antagonists to see which one is most useful for applications in imaging and therapy. They synthesized a series of neurotensin receptors with variable propylamine linker length and different chelators. From there, they performed radiolabeling reactions.
The team used western blot to determine expression in human lung cancer cell lines. It also assessed in vitro and in vivo stability of the antagonists as well as their binding affinity to lung cancer cells. Finally, the group used small-animal PET/CT imaging to evaluate the biodistribution properties of the agents included in the study.
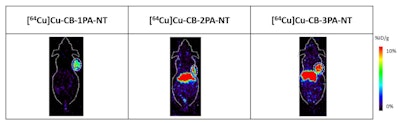 Representative PET imaging shows three leading compounds in lung cancer tumors in mouse models at 24 hours post injection at 10 %ID/g scale. Images courtesy of SNMMI.
Representative PET imaging shows three leading compounds in lung cancer tumors in mouse models at 24 hours post injection at 10 %ID/g scale. Images courtesy of SNMMI.The researchers confirmed via western blot that neurotensin receptors have high expression in the lung cancer cells. They found that one agent they developed, CB-2PA-NT, showed "good" binding affinity toward the lung cancer cells with half maximal inhibitory concentration (IC50) to be 3.038 nanometers.
Additionally, the team found that small-animal PET/CT demonstrated "prominent" tumor uptake, high tumor-to-background contrast, and long tumor retention up to 48 hours after injection.
The CB-2PA-NT agent showed 8.41% ID/g tumor uptake, which was maintained at 9.72% ID/g at 48 hours. The team also observed "moderate" liver uptake (7.72% ID/g at 48 hours) and low uptake in "most other organs." After comparison to other antagonist agents, the researchers identified CB-2PA-NT as the leading agent for further evaluation.
Ma said the findings suggest that the team's results show the potential for the agent to significantly expand the scope of precision medicine.
They are also collaborating with University of Wisconsin researchers to further explore the theranostic potential of the agent. The goal is to have first-in-human imaging performed with the agent, pending regulatory approval.