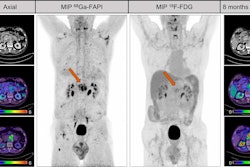
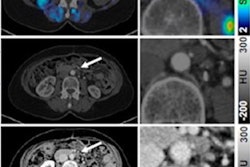
PET/CT with gallium-68 (Ga-68) FAPI radiotracer can predict responses to neoadjuvant chemotherapy in breast cancer patients, according to a study published November 2 in the Journal of Nuclear Medicine.
A team in Fuzhou, China, found that Ga-68 FAPI uptake on early PET/CT scans during chemotherapy indicated which patients were most likely to experience complete responses. This information can have a major impact on how patients are guided through treatment, according to the authors.
“Ga-68 FAPI-PET/CT parameters can rapidly provide feedback on tumor changes after only two cycles of chemotherapy,” wrote first author Ling Chen, MD, of the First Affiliated Hospital of Fujian Medical University, and colleagues.
Neoadjuvant chemotherapy is used to reduce tumors in patients to allow surgery or breast preservation and is also an important basis for adjusting follow-up adjuvant treatment plans, the authors explained.
In recent years, F-18 FDG-PET/CT has emerged as an option to monitor responses to NAC in patients based on glucose metabolism detected in tumors. While this approach has high sensitivity, it has a relatively low specificity in predicting complete responses, they added.
Conversely, Ga-68 FAPI is a relatively new radiotracer that binds to fibroblast activation protein (FAP) on the surface of cancer cells and has proven in early studies to be more specific than FDG tracer in these patients.
To further explore this potential, the researchers tested the approach in 22 patients who had newly diagnosed breast cancer confirmed by biopsy and who received NAC. All patients underwent Ga-68 FAPI-PET/CT scans at baseline (PET1), after two cycles of NAC (PET2), and one week before surgery (PET3).
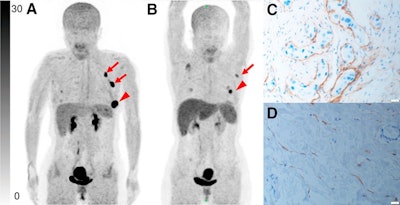 Representative Ga-68 FAPI-PET/CT images of 48-year-old woman who had left invasive ductal carcinoma and who achieved pathologic complete response (pCR). (A) Baseline maximum-intensity projection (MIP) image demonstrated primary lesion (red arrowhead; SUVmax, 26.72) and Ga-68 FAPI-avid lymph node in left axilla (red arrows). (B) MIP image after two cycles of neoadjuvant chemotherapy (NAC) showed that primary lesion (red arrowhead; SUVmax, 8.87) and axillary lymph nodes (red arrow) were reduced in size and radioactivity uptake. (C and D) Representative images of lab tissue staining for FAP before NAC showed strong FAP-positive staining in stromal cells (C), which was significantly decreased on tumor bed after six cycles of NAC (D). Image courtesy of the Journal of Nuclear Medicine.
Representative Ga-68 FAPI-PET/CT images of 48-year-old woman who had left invasive ductal carcinoma and who achieved pathologic complete response (pCR). (A) Baseline maximum-intensity projection (MIP) image demonstrated primary lesion (red arrowhead; SUVmax, 26.72) and Ga-68 FAPI-avid lymph node in left axilla (red arrows). (B) MIP image after two cycles of neoadjuvant chemotherapy (NAC) showed that primary lesion (red arrowhead; SUVmax, 8.87) and axillary lymph nodes (red arrow) were reduced in size and radioactivity uptake. (C and D) Representative images of lab tissue staining for FAP before NAC showed strong FAP-positive staining in stromal cells (C), which was significantly decreased on tumor bed after six cycles of NAC (D). Image courtesy of the Journal of Nuclear Medicine.
Seven patients (31.8%) achieved a pathologic complete response (pCR), and 15 (68.2%) had residual tumors. Thirteen patients (59.1%) showed concentric withdrawal (a shrink pattern) of the primary tumor, and nine (40.9%) showed diffuse withdrawal.
According to the analysis, absolute radiotracer standard uptake values (SUVmax) and tumor-to-background ratios (TBR) at PET2 and PET3 were lower in patients with pCR than in those without pCR. Moreover, a larger SUVmax at any time point was strongly associated with pCR (p < 0.05), the researchers found.
“Ga-68 FAPI-PET/CT has great potential for the early prediction of a pathologic response to NAC in [breast cancer],” the group wrote.
The authors noted limitations of the study, namely that it was a pilot prospective endeavor at a single institution with a limited sample size, and that further multi-institutional studies with larger samples are needed to validate the results.
Nonetheless, at present, there is no recognized method to accurately predict at an early stage of neoadjuvant treatment whether pCR can be reached and the results of this study warrant further research, they wrote.
“Future studies should consider cancer subtypes and chemotherapy regimens and further validate Ga-68 FAPI-PET/CT as an early response indicator to support its use as a better clinical tool for guiding patient treatment,” the researchers concluded.
The full article can be found here.