F-18 FDG-PET/CT scans may predict outcomes in patients with large B cell lymphoma undergoing chimeric antigen receptor (CAR) T-cell therapy, according to a presentation delivered November 30 at RSNA.
Doris Leithner, MD, of Memorial Sloan-Kettering Cancer Center in New York City, presented a study in which researchers explored whether F-18 FDG-PET/CT imaging features acquired prior to treatment with CAR T-cell therapy were related to patient outcomes. They found the imaging features were strongly associated with death, relapse, and disease progression.
“Our results demonstrate that quantitative PET imaging features in LBCL may serve as biomarkers for CAR T-cell therapy toxicity and likelihood of treatment failure,” Leithner said.
Large B-cell lymphoma (LBCL) is the most prevalent type of non-Hodgkin lymphoma, with about 50% of patients experiencing primary refractory or relapse after initial conventional treatments, Leithner explained. CAR T-cell therapy has provided new hope for these patients, yet up to 60% still experience disease progression within six months due to immune system reactions to the treatment.
“Because of this, and also due to the timing, financial, and infrastructural demands of CART T-cell therapy, we desperately need biomarkers that predict risk of treatment failure before CAR T-cell treatment,” Leithner said.
F-18 FDG-PET/CT scans are standard procedures for staging LBCL patients and assessing responses to conventional therapy, but its role in CAR T-cell therapy has not yet been fully explored, she added.
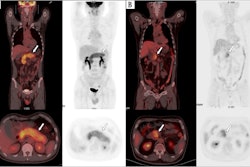
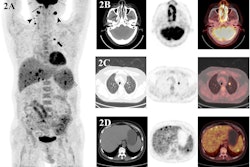
To that end, in this study, Leithner and colleagues evaluated whether F-18 FDG-PET/CT features – specifically, metabolic tumor volume (MTV), maximum standardized uptake value (SUVmax), and total lesion glycolysis (TLG) – could predict the likelihood of patient responses. These features are markers of the metabolic activity and size of tumors. The group analyzed associations between the features in 180 patients prior to CAR T-cell treatment and outcomes using univariable logistic regression and Cox regression models.
Out of the 180 patients (mean age 66, 121 male), 58% had achieved complete response and 21% had achieved partial responses 100 days after CAR T-cell treatment. Longer term, patients had a median progression-free survival (PFS) of 6.2 months and a median overall survival (OS) rate of two years.
According to the findings, increasing SUVmax, MTV, and TLG at the latest disease assessment in patients using F-18 FDG-PET/CT were strongly associated with shorter PFS and OS.
| F-18 FDG-PET/CT features associated with PFS and OS (HR = hazard ratios) | |||
|---|---|---|---|
| SUVmax | MTV | TLG | |
| Shorter PFS | HR = 1.28 | HR = 1.05 | HR = 1.06 |
| Shorter OS | HR = 1.33 | HR = 1.05 | HR = 1.06 |
Ultimately, the results demonstrate that quantitative PET imaging features in LBCL may serve as biomarkers for CAR-T cell therapy toxicity and the likelihood of treatment failure, Leithner said.
“These findings in one of the largest cohorts of LBCL patients may guide preventive interventions to increase the efficacy and safety of CAR-T cell therapy,” she concluded.