The European Commission has approved a $2.17 billion proposal by the Netherlands to fund construction of a new medical isotope production facility in Petten.
The new so-called Pallas reactor will replace the existing high flux reactor, one of the world leaders in the production of medical isotopes, in operation since 1961, the commission said, in a July 26 statement.
The new reactor is expected to start operating in the early 2030s. In addition, the proposal includes a nuclear health center for processing medical isotopes produced by the reactor into radiochemicals, which will then be further processed into radiopharmaceuticals that can be administered to patients for the diagnosis and treatment of several diseases, including cancer, the commission said.
The commission’s decision to green-light the proposal was subject to certain conditions that highlight the strain placed on U.S. medical isotope developers by foreign-subsidized competitors. The commission said that The Netherlands put in place sufficient safeguards to ensure that the aid has a limited impact on competition and trade.
“Our assessment has confirmed that the measures proposed by the Dutch authorities will limit any possible distortions of competition triggered by the public support,” said Margrethe Vestager, the commission’s executive vice president in charge of competition policy.
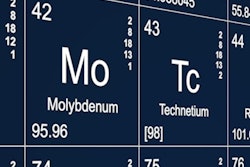
Last year, NorthStar Medical Radioisotopes cited foreign-government subsidized competitors as part of its reason for shutting down its molybdenum-99 (Mo-99) production facilities in Beloit, WI. Mo-99’s daughter decay chain isotope is technetium-99m (Tc-99m), which is used in an estimated 40,000 diagnostic nuclear medicine exams each day in the U.S.
In addition, in a recent interview with AuntMinnie.com, Greg Piefer, PhD, founder and CEO of Janesville, WI-based fusion energy company Shine Technologies, noted that foreign-subsidized competition made it difficult for the company to secure funding to launch its new approach of using fusion to produce medical isotopes.
“It’s our belief that some of these reactors are subsidized to about the point of only charging the medical isotope supply chain about 20% of what it costs to operate them,” he said.
Meanwhile, to help bolster U.S.-produced supplies, the U.S. Department of Energy recently awarded Shine $32 million to support production of Mo-99, with the company’s Chrysalis facility expected to achieve commercial production in early 2027.
Regardless, these developments may signal an end to past supply chain issues that have historically threatened patient access to critical diagnostic nuclear medicine tests and therapies in both Europe and the U.S.
Bertholt Leeftink, CEO of Nuclear Research and Consultancy Group, which runs the current facilities in Petten, noted in a news release that the commission’s decision is good news for patients with life-threatening diseases.
“With the arrival of the new Pallas reactor, the production of medical isotopes and the innovation of new applications for the treatment of, among other diseases, cancer will be guaranteed,” he said.