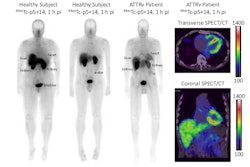
Attralus has received a breakthrough therapy designation from the U.S. Food and Drug Administration (FDA) for its PET radiotracer iodine-124 (I-124) evuzamitide (AT-01) for imaging cardiac amyloidosis.
The FDA granted the designation based on clinical data from Attralus-sponsored and investigator-initiated studies evaluating the use of I-124 evuzamitide PET in more than 200 trial participants, the company said.
Cardiac amyloidosis develops when amyloid proteins misfold and build up in the myocardium. This restricts cardiac function and can lead to heart failure and death. Currently, there are no FDA-approved diagnostic imaging agents for cardiac amyloidosis, Attralus noted.