SPECT/CT imaging can show over time how patients taking tafamidis for heart disease respond to the treatment, a group at Brigham and Women’s Hospital in Boston reported.
In a study of patients with transthyretin cardiac amyloidosis taking the medication, the researchers found that technetium-99m (Tc-99m) pyrophosphate (PYP) SPECT/CT metrics significantly decreased over an average period of 2.8 years. Meanwhile, patient echocardiograms and serum biomarkers remained unchanged, the group noted.
“Our findings suggest that improvements in quantitative Tc-99m PYP-SPECT/CT metrics, after transthyretin stabilization therapy, reflect different pathophysiology than captured by echocardiography, laboratory, and clinical parameters,” wrote Shilpa Vijayakumar, MD, and colleagues. The study was published August 10 in the Journal of Nuclear Cardiology.
Transthyretin cardiac amyloidosis (ATTR-CM) is a life-threatening disease that occurs when amyloid fibrils accumulate in the heart. These buildups occur when the liver produces misfolding transthyretin proteins, the authors explained. Tafamidis is a medication that binds to transthyretin to prevent it from misfolding, and thus stems the accumulation of amyloid fibrils.
SPECT/CT imaging can detect these amyloid deposits based on their uptake of radiotracers like Tc-99m PYP and is an established noninvasive technique for diagnosing the disease. Yet it is less clear whether the approach can longitudinally track disease progression or whether patients are responding to therapy, they noted.
To assess Tc-99m PYP-SPECT/CT for this purpose, the researchers enrolled 23 patients (average age, 75) with confirmed ATTR-CM and performed baseline scans as well as follow-up scans, with a median of 2.8 years between scans. All participants began transthyretin stabilization treatment (22 with tafamidis 61 mg once daily and one with diflunisal 250 mg twice daily) after the baseline scan and remained on treatment through their follow-up scan.
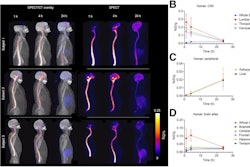
According to the analysis, on the follow-up Tc-99m PYP SPECT/CT images, visual grades decreased significantly (p = 0.003), with a significant reduction in multiple quantitative metrics, the group reported.
 Sagittal, coronal, and axial fused Tc-99m PYP-SPECT/CT images of a 93-year-old male at baseline and after two years of tafamidis 61 mg daily by mouth therapy. Tc-99m PYP images are shown in color and CT images are shown in grey scale. These images show a reduction in visual grade from an initial grade of 3 (myocardial uptake > rib uptake) to a follow-up grade of 1 (myocardial uptake < rib uptake). Quantitative metrics decreased significantly as well.Image and caption courtesy of the Journal of Nuclear Cardiology
Sagittal, coronal, and axial fused Tc-99m PYP-SPECT/CT images of a 93-year-old male at baseline and after two years of tafamidis 61 mg daily by mouth therapy. Tc-99m PYP images are shown in color and CT images are shown in grey scale. These images show a reduction in visual grade from an initial grade of 3 (myocardial uptake > rib uptake) to a follow-up grade of 1 (myocardial uptake < rib uptake). Quantitative metrics decreased significantly as well.Image and caption courtesy of the Journal of Nuclear Cardiology
Specifically, they observed significant reductions in maximum standard radiotracer uptake values (SUVmax: median change -0.75, p = 0.011), cardiac amyloid activity (CAA: median change -406.6; p < 0.001), and percent injected dose (%ID: median change -0.45, p < 0.001), a measurement of tracer uptake in a specific region of interest.
Additionally, echocardiographic parameters (global longitudinal strain, left ventricle mass index, left ventricle wall thickness), N-terminal pro-B-type natriuretic peptide, and estimated glomerular filtration rate remained stable, the group noted.
“Favorable changes in Tc-99m PYP myocardial uptake were observed in participants on transthyretin stabilization therapy, while echocardiographic parameters and biomarkers remained stable,” the researchers wrote.
Taken together, the findings imply that the decrease in tracer uptake in the hearts of treated patients reflects a molecular change indicating disease stabilization rather than regression of amyloid mass, the researchers suggested.
“It is also possible that molecular stabilization in the myocardium appears earlier in the course of therapy and may herald future improvements in cardiac structure, function, and clinical outcomes,” they wrote.
Since this was a small study, further research is needed to understand its implications, the researchers concluded.
The full study is available here.