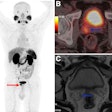
Berlin-based Ariceum Therapeutics has received investigational new drug clearance from the U.S. Food and Drug Administration (FDA) to launch a phase I/II clinical trial of a radiolabelled peptide that targets small cell lung cancer or Merkel Cell Carcinoma (MCC).
The so-called “SANTANA-225” trial will assess the safety, tolerability, preliminary efficacy, and recommended dose of actinium-225 (Ac-225) SSO110 in patients with extensive-stage SCLC or MCC who are on first-line maintenance therapy with checkpoint inhibitors. Ac-225 SSO110 is being developed together with a companion gallium-68 (Ga-68) SSO120 radiotracer as a “theranostic pair,” the company noted. The company is working with partners and clinical sites in the U.S. and other countries to commence recruitment of patients in Q1 2025.
In other recent news, Ariceum expanded its global supply agreements for Ac-225, as well as lutetium-177, which can also be used to radiolabel SSO110.