Researchers in France have developed a PET imaging approach for longitudinal tracking of the virus that causes COVID-19.
In a study in nonhuman primates, the approach visualized SARS-CoV-2 in the lungs and the brain three months following infection, noted Alexandra Detrille, PhD, of Paris-Saclay University, and colleagues.
“The technology we developed offers a comprehensive assessment of SARS-CoV-2 distribution in vivo and provides a promising approach for the non-invasive study of long-COVID pathophysiology,” the group wrote. The study was published March 21 in Nature Communications.
While available treatments and vaccines have reduced the mortality rate from COVID-19 globally, the spread and evolution of the SARS-CoV-2 virus are ongoing processes, the authors explained. Specifically, despite extensive research, the long-term impact of infection is still poorly understood and requires further investigation, they noted.
To that end, the group first created a radiolabeled monoclonal antibody PET radiotracer that targets a spike protein on the SARS-CoV-2 virus. In in vitro lab studies over two weeks, the zirconium-89 (Zr-89) COVA1-27-DFO tracer maintained a high affinity for its target, the spike protein, both in the wildtype and delta variants of the virus.
Next, the researchers evaluated the uptake of the Zr-89 COVA1-27-DFO radiotracer in two convalescent macaques exposed to the SARS-CoV-2 delta variant three months prior to whole-body PET/CT imaging, as well as in two uninfected animals.
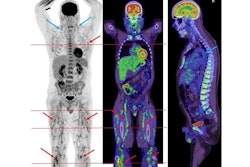
The group detected Zr-89 COVA1-27-DFO uptake by the lungs of one convalescent animal, along with CT ground-glass opacity. Tracer uptake on PET/CT was higher in lung lesion regions than in nonlesion lung areas, and significantly higher than in uninfected animals, according to the findings.
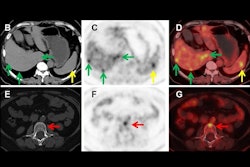
 Representative PET/CT fusion axial view of the sinus of one convalescent animal (CM2) with the corresponding quantification (b, c) of the mean and maximum Zr-89 COVA1-27-DFO PET signal in the brain of all animals normalized to that at day 1 post-injection. (d) Representative CT lung lesion, indicated by the yellow circle, and its associated PET signal (e), indicated by the white circle, on day 7 post injection. (f) Corresponding quantification of the maximum PET signal in the lesional (gray background, n = 6 areas) and nonlesional areas (square symbols, white background, n = 6 areas) on day 7 post injection compared with that for naïve animals (black symbols (n = 7 areas). (g) Axial view of the Zr-89 COVA1-27-DFO PET signal in the brain, indicated by the white arrows, at day 3 post injection. Corresponding quantification (h, i) of the maximum and mean Zr-89 COVA1-27-DFO PET signal in the brains of all animals normalized against that on day 1 post injection. Mann Whitney two-sided t-test, *: p = 0.014, **: p = 0.008. Convalescent + COVA animals are indicated in green and the mock + COVA animals in black. Data are presented as individual values and the associated mean and SD. Source data are provided as a Source data file. Image and caption available for republishing under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of Nature Communications.
Representative PET/CT fusion axial view of the sinus of one convalescent animal (CM2) with the corresponding quantification (b, c) of the mean and maximum Zr-89 COVA1-27-DFO PET signal in the brain of all animals normalized to that at day 1 post-injection. (d) Representative CT lung lesion, indicated by the yellow circle, and its associated PET signal (e), indicated by the white circle, on day 7 post injection. (f) Corresponding quantification of the maximum PET signal in the lesional (gray background, n = 6 areas) and nonlesional areas (square symbols, white background, n = 6 areas) on day 7 post injection compared with that for naïve animals (black symbols (n = 7 areas). (g) Axial view of the Zr-89 COVA1-27-DFO PET signal in the brain, indicated by the white arrows, at day 3 post injection. Corresponding quantification (h, i) of the maximum and mean Zr-89 COVA1-27-DFO PET signal in the brains of all animals normalized against that on day 1 post injection. Mann Whitney two-sided t-test, *: p = 0.014, **: p = 0.008. Convalescent + COVA animals are indicated in green and the mock + COVA animals in black. Data are presented as individual values and the associated mean and SD. Source data are provided as a Source data file. Image and caption available for republishing under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of Nature Communications.
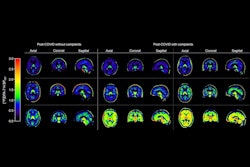
In addition, the researchers found a visible PET signal in certain regions of the brains of both convalescent animals, with much higher maximum standard uptake values than in uninfected animals (ratios of 0.8 and 0.63 vs. 0.43 and 0.48).
“The Zr-89 COVA1-27-DFO radiotracer allows detection of the PET signal for SARS-CoV-2 spike antigen up to three months post initial infection in the lungs and brains of COVID-19 convalescent animals,” the group wrote.
The approach provides a powerful innovative tool to study COVID-19 pathophysiology and viral spreading, the authors suggested. It could also provide insights for long-COVID studies by studying antigen persistence in key organs such as the brain and lungs, as well as for potential treatment and vaccine efficacy studies, they added.
Ultimately, the study was limited by the number of animals in the experiments, which reduced the statistical significance of the results, the authors noted. Further Zr-89 COVA1-27 PET imaging studies could also be conducted on animals exposed to other SARS-CoV-2 variants of interest or using other exposure routes (e.g., aerosols), as these conditions may better reproduce the natural infection, they suggested.
“To better investigate the presence of the virus in the brain in the future, a nanobody-based radiotracer could be used to increase [blood-brain barrier] penetration,” the group wrote.
The full study can be found here.