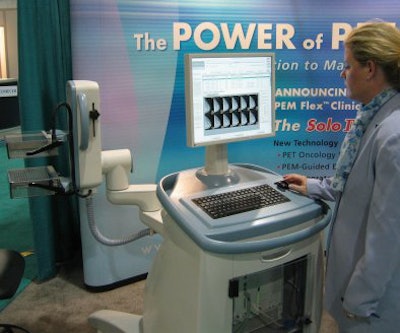
Visitors to the Society of Nuclear Medicine (SNM) booth of PET developer Naviscan PET Systems of San Diego got a look at a new version of the company's positron emission mammography (PEM) system, which has received a design makeover.
The new system, called PEM Flex Solo II, has all the features of the older PEM Flex Solo system but in a more attractive and ergonomic design, according to Tom Watlington, president of the San Diego company. The new unit also features the detector assembly attached to the technologist workstation via an articulating arm, whereas previously the two were separate components.
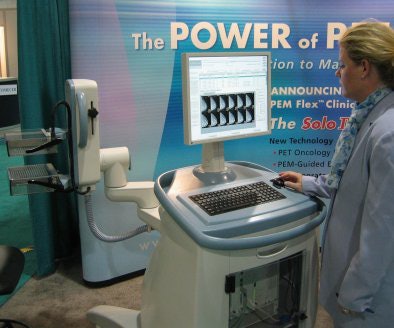 |
| Naviscan unveiled a new design for its positron emission mammography (PEM) technology. |
Naviscan has also made upgrades to the graphical user interface of the system's software, as well as to the unit's sensitivity, and hopes to have support for DICOM 3.0 available by the end of the year. PEM Flex Solo II will carry a list price of $500,000, about the same price as the PEM Flex Solo I system, and the first units will be available for clinical trials in September.
Naviscan also demonstrated a biopsy localization feature as a work-in-progress for both the Solo I and Solo II systems. Users will be able to take a standard biopsy device and add a probe that is loaded with a germanium radioisotope that can be tracked on a PET scan. The probe will enable real-time lesion localization, an important feature for any breast imaging modality.
Naviscan is also working on launching a multicenter clinical trial that will compare PEM technology to breast MR. The firm hopes that the trial will demonstrate that PEM has a higher specificity than breast MR, which is notoriously plagued by false positives. The two technologies have about the same sensitivity, according to Watlington. Patient collection for the trial will begin in July or August, he said.
By AuntMinnie.com staff writers
June 7, 2006
Related Reading
Naviscan raises $6.5 million, moves headquarters, November 29, 2005
Road to RSNA, Naviscan PET Systems, November 3, 2005
Road to RSNA, Naviscan PET Systems, November 8, 2004
Road to RSNA, Naviscan PET Systems, November 19, 2003
FDA clears Naviscan PET scanner, September 30, 2003
Copyright © 2006 AuntMinnie.com