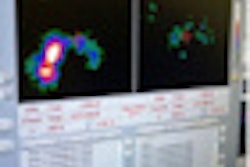
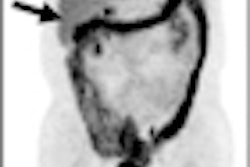
GE Healthcare said that interim results of a recently completed study showed that its DaTscan radiopharmaceutical (ioflupane I-123) could provide useful objective evidence for patients with clinically uncertain Parkinsonian syndrome.
In data from a multicenter, open-label, randomized clinical trial presented at the 2011 American Academy of Neurology (AAN) meeting in Honolulu this week, interim results showed that more patients in the group receiving DaTscan SPECT imaging had changes from baseline diagnosis, and their physicians had a higher mean confidence of diagnosis, compared with the group that didn't receive DaTscan, GE said.
Furthermore, in patients with a management plan at baseline, those who received DaTscan had significantly more management changes 12 weeks after randomization than those who did not receive DaTscan. The full study will evaluate patients through one year from baseline, according to GE.