There may be an alternative to non-small cell lung cancer (NSCLC) diagnosis and staging using FDG-PET: low-cost SPECT imaging with a new molecular imaging agent, according to research from MD Anderson Cancer Center and biopharmaceutical company Cell>Point.
PET imaging using the radiotracer F-18 FDG is the current standard for diagnosis and staging of patients with non-small cell lung cancer. The number of PET scanners available, however, is currently lower than ideal. In the U.S., for example, it's estimated that only one out of every four patients who could benefit from staging receives a PET scan. In Europe, this gap is more than tenfold higher.
In the late 1990s, when PET and FDG were first approved for oncology applications, there was already concern that the high cost of PET devices would limit the number of sites that could utilize this technology. As such, researchers at MD Anderson began work on creating an alternative imaging agent that would make access to cancer staging more widely available.
The MD Anderson team, working in collaboration with U.S. biopharmaceutical company Cell>Point, has now developed just such a molecular imaging agent. The new agent, technetium-99m (Tc-99m) ethylene dicysteine glucosamine (ECG), can be imaged using SPECT systems, which are lower in cost and have a far greater installed base.
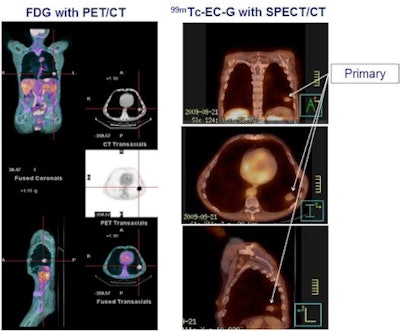 |
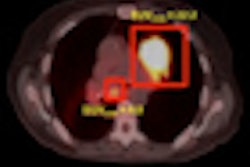
| Phase II result: Tc-99m ECG (right) is at least equivalent to FDG (left) in detecting primary NSCLC tumors (100% concordance). Image courtesy of medicalphysicsweb. |
Phase II clinical trials examining NSCLC diagnosis with ECG have now been completed, and results were presented at the Society of Nuclear Medicine (SNM) annual meeting in San Antonio in June. "The first application focused on lung cancer because it represents approximately 80% of all staging procedures performed with FDG," explained Dr. David Rollo, president of Cell>Point.
ECG versus FDG
The phase II trial compared the diagnostic and staging accuracy of ECG-SPECT/CT with that of FDG-PET/CT in 22 patients with biopsy-proven NSCLC. Standard protocols were used for FDG imaging. The ECG protocol involved performing SPECT/CT three hours after injection of 1 mg of ECG labeled with 25 mg of Tc-99m. The study included SPECT/CT systems from all three major vendors at six participating sites.
Independent expert readers at a core lab assessed the images for location, size, and confidence that the detected lesions were cancer. For primary lesions, results showed 100% concordance between ECG and FDG on all three parameters, independent of the SPECT device used. In 17 patients with metastatic lesions, 90% agreement was seen for location, size, and confidence that detected lesions were metastases. No adverse drug-related events were reported.
The Cell>Point team also examined the uptake mechanism of the two radiotracers. Uptake of Tc-99m ECG increases in cells undergoing rapid regeneration, thus the agent targeted the tumor, with minimal localization in infection or inflammation, no normal-brain or bone uptake, and slight cardiac uptake. Increased FDG uptake occurs in cells with increased glucose metabolism, resulting in its localization in tumor, inflammation, normal brain, and normal cardiac tissue.
"Because ECG is not taken up in inflammation or infection, unlike FDG, it has the potential benefits of fewer false positives and the potential to be used to assess efficacy of chemotherapy during therapy," Rollo said.
Production matters
According to Cell>Point, another advantage of ECG is that it can be delivered to the clinic by a nuclear pharmacy as a unit-dose vial containing enough radiolabeled ECG to scan one patient. Prior to delivery, a nuclear pharmacy adds Tc-99m to the ECG vial, shakes it, and the radiolabeled agent is then ready to inject into the patient (a process known as "shake and shoot") at the imaging site.
The Tc-99m is created upon decay of molybdenum-99 (Mo-99), which has a half-life of 66 hours and is available on a 24-hour basis from a Mo-99/Tc-99m generator. Alternatively, many hospitals have their own Mo-99/Tc-99m generator and their own licensed radiopharmacist who can radiolabel the ECG onsite on a per-need basis.
FDG, on the other hand, is delivered as radiolabeled agent and injected into the patient without further preparation. F-18 decays with a half-life of 110 minutes, thus FDG-PET requires onsite or nearby generation of F-18 using a cyclotron.
Next step
With the phase II trial completed, Cell>Point has now prepared the phase III protocol and is awaiting U.S. Food and Drug Administration (FDA) assessment. "We are simultaneously beginning the recruiting process for up to 45 sites in the U.S. and Canada," Rollo said. "It is our plan to have the final FDA-approved protocol in place soon and to begin the phase III in Q4 2011."
Rollo added that animal imaging studies have shown a similar ECG uptake mechanism for all cancer types studied, including breast, lymphoma, liver, bone, brain, colon, and prostate cancers.
"Ultimately, we plan on conducting confirmatory trials for other cancer types, beginning with breast and lymphoma," he told medicalphysicsweb.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.