Contrast agent developer Bracco Diagnostics anticipates that shipments of its CardioGen-82 cardiac PET radiopharmaceutical will resume by the end of the first quarter, after the company voluntarily recalled the product six months ago.
Bracco and the U.S. Food and Drug Administration (FDA) have collaborated since last July to determine why three patients from two different facilities had increased levels of radiation in their bodies following PET scans in which CardioGen-82 (rubidium-82 chloride injection) was administered. The levels were discovered after the patients set off radiation detectors when crossing the border between the U.S. and Canada.
Following the FDA's July announcement that healthcare professionals not use CardioGen-82, Bracco voluntarily recalled the tracer. The company then contacted customer sites that used the radiopharmaceutical between January 2011 and July 2011 to gather additional information about any incidents of unexpected exposure. Bracco also conducted a review of all CardioGen-82 components and its manufacturing process.
The FDA theorized in its July communiqué that the increased radiation was due to strontium isotopes that may have been inadvertently injected into patients because of an alleged "strontium breakthrough" problem with CardioGen-82.
Bracco's probe, however, found that all CardioGen-82 generators had strontium-82 and strontium-85 levels "well within specification limits set forth in the prescribing information" and its generators were not to blame for the patients' excess radiation levels, according to a December 29 letter the company sent to its customers. In addition, data from customer sites showed no other patients with higher-than-expected radiation levels.
"It was determined that it was not an issue with the product; it was how [the product] was used," Kim McDaniel, Bracco's senior director of nuclear medicine sales and market support, told AuntMinnie.com. "Based on all of that information we presented, [the FDA] voiced their support of our phased reintroduction of the product back to the market with enhanced labeling, but it is the same product."
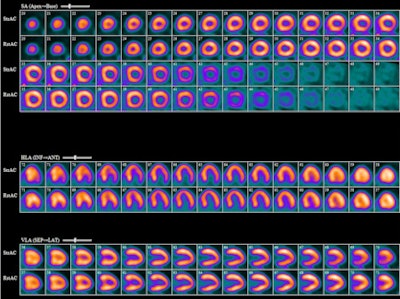 |
| With the help of CardioGen-82, mild degrees of ischemia are discovered through PET imaging. Image courtesy of Dr. Sharmila Dorbala, director of nuclear cardiology at Brigham and Women's Hospital in Boston. |
Bracco is currently working with the FDA to finalize the labeling information for the tracer. McDaniel said it is too soon to know the final language of the labeling.
The company also plans to initiate a new quality control data repository and monitoring program when CardioGen-82 returns to the market. In addition, Bracco's field nuclear medicine team will begin to contact facilities later this month to discuss and initiate quality control measures and staff training to ensure the proper use of CardioGen-82.
"We proposed to the FDA a controlled and phased reintroduction of CardioGen-82 generators to user facilities, with data collection and evaluation of the actual field use of each generator beginning Q1 2012," the company's customer letter stated.
Bracco expects to send more information regarding generator availability to its customers by February 1.