GE Healthcare touted results from four pooled brain biopsy studies and a brain autopsy study of its investigational PET amyloid imaging agent, F-18 flutemetamol, that confirm the agent's potential to detect beta amyloid plaque, a pathology associated with Alzheimer's disease.
These data are being presented as part of the Emerging Science Program at the American Academy of Neurology's annual meeting in New Orleans and support an application that the company is preparing for regulatory approval of flutemetamol.
The pooled analysis included 49 patients receiving flutemetamol before or after brain biopsy during shunt placement or intracranial pressure measurement, as well as 68 autopsy subjects, to establish the presence of brain amyloid pathology.
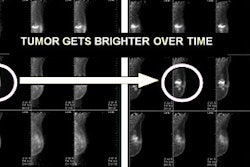
For patients with biopsy tissue samples, the study found that flutemetamol detected beta amyloid with a pooled sensitivity of 93% and pooled specificity of 100%, according to GE. In autopsied subjects, the agent detected beta amyloid with a sensitivity of 86% and specificity of 92%.
The accumulation of beta amyloid in the brain is believed to contribute to the degeneration of neurons in Alzheimer's disease and is one of several pathological characteristics implicated in its development, GE said.