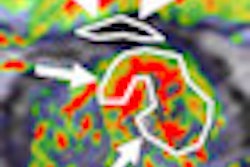
Radiopharmaceutical developer NuView Life Sciences is touting a phase I feasibility study of its NLS-VPAC1 breast cancer imaging agent.
In the study, NLS-VPAC1 (labeled with the radioisotope copper-64) with PET and positron emission mammography (PEM) was used in 19 patients. The agent identified all 20 malignant lesions in the patient group, regardless of hormonal status, and all four sentinel lymph nodes.
The study was presented at last month's World Congress on Ga-68 in Chandigarh, India, by Mathew Thakur, PhD, professor of radiology and director of radiopharmaceutical research and molecular imaging at Thomas Jefferson University.
NuView said it owns exclusive worldwide commercialization rights for NLS-VPAC1.
According to the company, NLS-VPAC1 imaging can be performed within 15 minutes of injection and does not require patient fasting or monitoring of glycemic levels. Due to the tumor uptake rate, NuView is also investigating the use of NLS-VPAC1 labeled with gallium-68, which has a shorter half-life.
The company plans to initiate a multicenter phase II trial in breast cancer in 2013. In addition, NuView and Thakur have been awarded a $2.6 million grant from the U.S. National Institutes of Health (NIH) to conduct a phase I feasibility study with NLS-VPAC1 in prostate cancer patients. Initiation of that trial is planned for the fourth quarter of 2013.