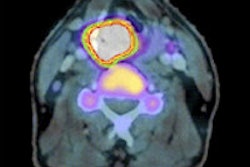
Radiopharmaceutical developer Navidea Biopharmaceuticals has launched its Lymphoseek nuclear medicine imaging agent in the U.S.
The U.S. Food and Drug Administration (FDA) approved Lymphoseek in March for use in lymphatic mapping procedures for patients with breast cancer and melanoma. It has been priced at $300 per patient procedure, according to Navidea.
Navidea said it will distribute the agent through its distribution partner, Cardinal Health.