Radiopharmaceutical developer Navidea Biopharmaceuticals has enrolled the first participant in a phase III trial of its NAV4694 PET radiopharmaceutical.
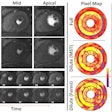
The trial is designed to assess the safety and efficacy of the investigational PET imaging agent to detect the presence or absence of beta amyloid in end-of-life subjects with and without dementia by correlating PET image findings during life with those of brain tissue upon autopsy after death.
The trial will enroll up to 275 end-of-life individuals in two cohorts. One group will include subjects diagnosed with probable Alzheimer's disease or other forms of dementia, while a second group will include volunteers with no signs of dementia.