Radiopharmaceutical firm Jubilant DraxImage has notified customers that its supply of a technetium-99m-based imaging agent will not be replenished until mid-October.
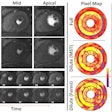
The DraxImage MAA (macroaggregated albumin) kit for the preparation of technetium-99m (Tc-99m) is used to evaluate pulmonary perfusion and may be used in adults to assess the efficacy of peritoneovenous shunts to drain fluid from the peritoneum, which lines the abdominal cavity, into veins.
In a letter dated September 6, the company noted that the shortage issue dates back to the middle of last year, when one of its suppliers ceased production.
Jubilant DraxImage then validated a new supplier and continued production, but the firm subsequently decided that manufacturing improvements were the best approach to maintain MAA supply and quality standards. Those manufacturing improvements are underway.
"Unfortunately, this has taken longer than originally anticipated and, therefore, has impacted our ability to supply our customers," the letter stated. "We have notified, as required, the Drug Shortages Division of the Food and Drug Administration of this situation."
The commercial manufacturing of MAA has several "critical process steps," which need precise manufacturing control, the company added. "Because of these challenges, a number of manufacturers have elected to cease production leaving us as the sole North American supplier."