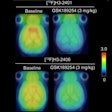
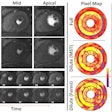
The Medical Imaging and Technology Alliance (MITA) cited progress in the U.S. Centers for Medicare and Medicaid Services' (CMS) final decision on beta-amyloid PET coverage; however, the organization said it was disappointed by the scope.
On September 27, CMS issued a final decision approving beta-amyloid PET under the agency's coverage with evidence development (CED) protocol for patients enrolled in an approved clinical study.
Although MITA appreciates that CMS is offering beta-amyloid PET to some Medicare beneficiaries, the group is disappointed there will not be coverage for beta-amyloid PET without CED for patients with suspected dementia or Alzheimer's disease who meet specific appropriate-use criteria.
The organization will continue to work with CMS toward a substantive, feasible CED program that will support broader access for Medicare patients, according to Gail Rodriguez, executive director of MITA.