Radiopharmaceutical vendor Navidea Biopharmaceuticals is promoting results from a clinical trial of its Lymphoseek imaging agent.
Results from its phase III NEO3-06 trial of Lymphoseek for carcinoma of the head or in the mouth are positive enough for the company to file a supplemental new drug application (sNDA) with the U.S. Food and Drug Administration (FDA) by the end of the year.
Navidea said it also anticipates action related to the European Union marketing authorization application (MAA) for Lymphoseek by the end of 2013.
The NEO3-06 trial is part of Navidea's global strategy to expand Lymphoseek (technetium-99m tilmanocept) to multiple cancer types to help physicians improve their accuracy in gauging the extent of disease, the company said.
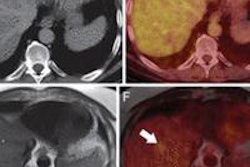
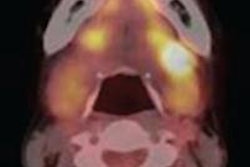
Navidea previously announced that the NEO3-06 study was closed after having met the primary false-negative rate efficacy end point of accurately identifying sentinel lymph nodes (SLNs), compared with the gold standard of removing all lymph nodes during multiple-level nodal dissection surgery of the head and neck.
The completed full study report states that Lymphoseek also met all other prespecified study end points, including sensitivity, negative predictive value, and overall accuracy relative to the pathology status of nonsentinel lymph nodes, the company said.