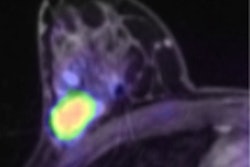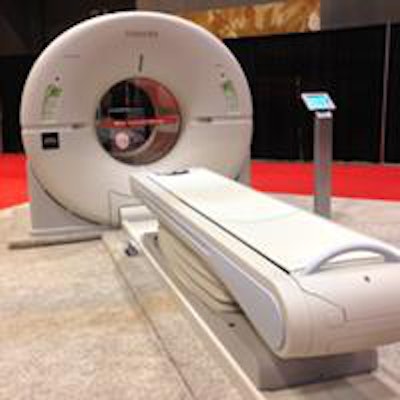
For the first time in some seven years, Toshiba America Medical Systems is officially a player again in the U.S. nuclear medicine market with its newly introduced and recently cleared Celesteion PET/CT system.
The company received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in mid-June to market the hybrid midlevel PET/CT system, which is designed for diagnostic and radiation oncology imaging, including tumor detection, treatment evaluation, and CT simulation.
With the FDA OK, the company is poised to go head-to-head with longtime market mainstays GE Healthcare, Philips Healthcare, and Siemens Healthcare.
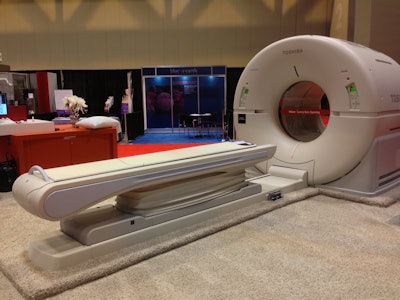 Toshiba debuted the Celesteion PET/CT system earlier this month at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
Toshiba debuted the Celesteion PET/CT system earlier this month at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.Reasons for re-entry
One of the main reasons Toshiba returned to the U.S. nuclear medicine and molecular imaging market is because the company saw growth in oncology procedures, said Satrajit Misra, senior director of Toshiba's CT business unit. PET, of course, has long been a staple of oncology imaging, tumor staging, and patient follow-up.
"PET reimbursement, unlike many other modalities, has remained very steady over multiple reimbursement cuts," he said. "The number of PET scans that are reimbursed for follow-up has actually gone up, so there is a very positive trend in PET imaging."
As the U.S. population continues to age and people live longer, the overall number of individuals developing cancer continues to grow.
"It is a very expensive disease to manage," Misra said. "A lot of CT imaging, for example, is oriented around cancer staging and cancer diagnosis as well. [Celesteion] allows us to offer a more comprehensive, multimodality system in the oncology space."
Now that Celesteion is approved for marketing, Toshiba plans to take orders in the next few weeks and begin shipments in the latter part of this year. "We believe that we should very quickly have a few installations all across the country," Misra added.
'A little vulnerable'
He conceded that Toshiba might have been "a little vulnerable" in recent years by not having an offering in nuclear medicine. Toshiba has CT and MRI scanners, which helped the company keep a presence in oncology, but the lack of a nuclear medicine imaging system could have hindered the consummation of a few big contracts.
"Yes, it was a slight disadvantage in not having a complete oncology portfolio in large deals," Misra said, "but I think this product has us better poised to address those needs."
During its absence from the U.S. nuclear medicine market, Toshiba continued to sell PET/CT systems in Japan; Celesteion is a new product for both the U.S. and Japanese markets. Whether the company will bring the hybrid scanner to other parts of the world is still undecided.
"We are closely evaluating other geographies that we may want to enter, but we want to play to our strengths and focus initially on the two primary markets that we feel will drive business growth for us," Misra said.
Toshiba also currently markets a SPECT system in Japan, but there has been no decision yet on whether to bring the gamma camera to the U.S. to expand its nuclear medicine portfolio. "We are keeping our options open at this point in time," Misra added.
Homegrown technology
Celesteion's PET technology was developed at the Toshiba Medical Research Institute USA (TMRU) in Chicago.
"It has been about a five-year project," said Timothy Nicholson, Toshiba's senior manager for CT market development. "The CT side is a product that Toshiba has sold for approximately eight years, the Aquilion large-bore CT."
The hybrid system features a 90-cm CT bore and 88-cm PET bore to give patients a feeling of openness. The PET component also offers 450-picosecond time-of-flight resolution, while the CT scanner has a 0.5-second rotation with 0.5-mm 32-slice detectors.
Celesteion also has 70-cm CT and PET field-of-views and an 85-cm CT extended field-of-view; in addition, Toshiba's adaptive iterative dose reduction (AIDR) 3D CT dose reduction technology is a standard feature. The system also conforms to the Medical Imaging and Technology Alliance (MITA) XR-29 Smart Dose Standard.
Toshiba is now hoping for positive results in the U.S. with its new PET/CT system. "We want to start affecting the standard of care from a patient experience and dose reduction standpoint," Misra said. "We want to drive the market to design systems that are oriented around the patient to make the patient experience much better, and drive new standards in dose reduction for hybrid imaging modalities."