Molecular imaging developer Gamma Medica is hoping that 2015 will be a watershed year in its resurrection, some 21 months after emerging from Chapter 11 bankruptcy. The company has refreshed its technology and is building its installed base as it regains its footing.
In March 2013, the previous iteration of Gamma Medica sold its molecular breast imaging (MBI) business and preclinical imaging segments two separate buyers. The firm's preclinical business was acquired by Northridge Tri-Modality Imaging for $2.5 million, while the LumaGem MBI technology was sold to an investment group led by existing Gamma Medica investor Psilos Group Managers for $3.1 million.
Psilos sought to continue the commercialization strategy for LumaGem and relaunch the company. Since then, the new Gamma Medica has worked to remake the company and upgrade the LumaGem device and its MBI technology.
 Jim Calandra, president and CEO of Gamma Medica.
Jim Calandra, president and CEO of Gamma Medica."In a lot of ways, it is a classic turnaround, and we are at the final tactical stage," said Jim Calandra, president and CEO of Gamma Medica. "We are at the stage of a normal start-up trying to measure the commercial adoption of our technology."
Calandra also served as CEO of Gamma Medica prior to the bankruptcy and company split. The LumaGem MBI system is cleared by the U.S. Food and Drug Administration (FDA) for use in women with complex breast anatomy, such as dense breast tissue, when screening mammograms and ultrasound scans may miss early cancers.
LumaGem upgrades
Since the transaction, Gamma Medica has made a concerted effort to improve the LumaGem technology. For example, the company ratcheted up specification requirements for its cadmium zinc telluride (CZT) digital detectors.
"The more uniform the crystals are in the camera, the better the camera performs," Calandra said "We also get much better images at lower radiation dosage and/or lower scan time."
A software upgrade is due for release in the first quarter of 2015 to create a "refreshed" interface with additional functionality. Gamma Medica is also about a year into developing biopsy guidance capability.
"We have a prototype, we have tested it, and we are comfortable that we are making good progress," Calandra said. "At the moment, it looks like a late 2015 product release."
When Calandra and colleagues acquired Gamma Medica's MBI assets in 2013, there were 18 orders for LumaGem, but two potential buyers canceled. "At the time of the acquisition, we also acquired five open orders and we kept all five," he added. "So we have started to regrow the base back."
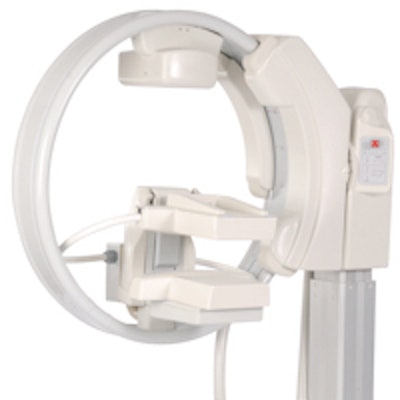
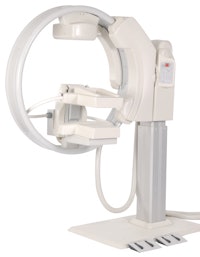 Gamma Medica has made several improvements to LumaGem, including bolstering the mechanism that controls the camera elevation. Image courtesy of Gamma Medica.
Gamma Medica has made several improvements to LumaGem, including bolstering the mechanism that controls the camera elevation. Image courtesy of Gamma Medica.The company has installed five units since July, bringing the total installed base to 21 (20 systems in the U.S. and one in Italy), and anticipates three or four more shipments before the end of the year.
Challenges for 2015
"What is critical for us now is driving sales," Calandra said. "I'd like to say the first part of 2015 is critical, but there is so much going on in healthcare that it's hard to know where we really are."
Specifically, he cited changes caused by the Affordable Care Act, turmoil from the consolidation of practices and providers, and uncertainty about reimbursement as reasons for the bouts of buying paralysis at healthcare facilities.
"When you're asking a customer to make a meaningful capital investment in a piece of equipment -- and they are not accustomed to having this kind of technology in their breast imaging workflow -- it is easy to see how they might say, 'Not now,' " he said.
At least reimbursement for MBI scans is not an issue. When the U.S. Centers for Medicare and Medicaid Services (CMS) released its reimbursement rates last year, the agency rolled the two CPT codes for localization with a gamma camera into one code. The universal reimbursement for the revised CPT code was 27% greater than the previous payment. The national average for Medicare reimbursement is now $383.
"We made out pretty well," Calandra added.
Molecular breast imaging technology, however, is battling breast MRI in the marketplace. But while clinicians tend to be more familiar with MRI, MBI is one-third the cost of MRI, takes up less space, and has considerably lower service costs, Calandra noted.
"Imaging time is one-half, and the real benefit is that the true-positive biopsy rate with molecular breast imaging is nearly twice what it is on MRI," he added.
Company relocation
One key move in Gamma Medica's resurrection came about two years ago, when the company moved from Northridge, CA, to Salem, NH. Salem is just a few miles over the New Hampshire-Massachusetts state line and affords Gamma Medica the opportunity to tap the wealth of medical and technological talent in the greater Boston area.
Calandra, who is a 30-year New Hampshire resident himself, said the cost of doing business is lower in the Granite State than in Massachusetts, and New Hampshire has no personal state income tax, which greatly benefits employees.
There are 31 people on staff, only three of whom are holdovers from the previous company. The only function outsourced by Gamma Media is the assembly of the gantry at a manufacturing facility in Ohio. Everything else is handled in Salem.
Gamma Medica is also only eight miles from Philips Healthcare's medical imaging campus in North Andover, MA.
Coincidentally, Gamma Medica inked a partnership with Philips this month. The company will offer Philips' MicroDose SI full-field digital mammography system as a complement to its LumaGem MBI system. The pact will also allow Gamma Medica to provide breast imaging suites with the option to purchase both technologies.
In addition, Gamma Medica this month aligned with Volpara Solutions, which offers screening protocols based on a woman's breast density and individual risk factors for cancer.
On the business side, Gamma Medica received a financial boost from two sources in October. Hercules Technology Growth Capital invested $6.5 million in the company, while current investor Psilos anted up $5 million. Gamma Medica plans to use the funding to accelerate sales and marketing efforts and to promote the adoption of its MBI technology.
"There will come a time when [MBI] has to be commercially viable and stand on its own two feet," Calandra said. "At the end of the day, success in a commercial endeavor is measured by the response from the intended customer and the market. We will have to measure that eventually. Hopefully, we'll get that measurement soon."