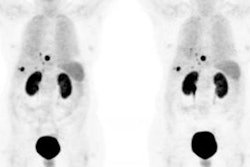
PET/CT scans with the investigational radiopharmaceutical gallium-68 (Ga-68) DOTATOC homed in on neuroendocrine tumors and changed patient management in a number of cases, according to a study presented at this week's Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
Ga-68 DOTATOC binds to somatostatin receptors in neuroendocrine cells; by binding more strongly to cells with higher cellular proliferation, Ga-68 DOTATOC can highlight potentially malignant areas.
A research team from the University of Iowa, led by Dr. Yusuf Menda, administered Ga-68 DOTATOC to 36 patients with metastatic neuroendocrine tumors but an unknown primary tumor site. Such metastases can often mask primary tumor sites, a phenomenon that is particularly problematic with neuroendocrine tumors.
Of the 36 patients, 29 had PET/CT scans that revealed strong-binding metastases with high affinity for the radiopharmaceutical, and the location of the primary tumor was found in 19 patients.
Further imaging confirmed the primary tumor site in three patients and histological exams confirmed the site in eight patients. Five suspected primary tumors remained undefined, and no primary tumor site was found in seven patients. The researchers noted that 28% of patients underwent a major change in patient management as a result of Ga-68 DOTATOC scans.
While Ga-68 DOTATOC is still investigational in the U.S., the radiotracer is being researched in clinical trials, which, combined with experience from published studies in Europe, could lead to a commercially available product in one to two years, according to Menda.
In 2013, Ga-68 DOTATOC was granted orphan drug status by the U.S. Food and Drug Administration (FDA). The orphan drug program is designed for pharmaceuticals for rare conditions, for which their developers are not expected to recoup the costs of development. The FDA waives drug user fees for orphan drugs, and developers can also receive tax credits.