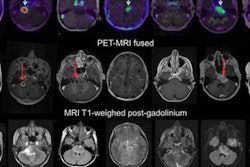
BALTIMORE - Clinicians may soon be able to add PET/MRI with F-18 fluorodopa (FDOPA) to their imaging arsenal to evaluate the efficacy of the angiogenesis inhibitor bevacizumab for pediatric brain tumors, according to a study presented on Monday at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
Researchers from Washington University in St. Louis found that using the amino acid tracer FDOPA with PET/MRI is a feasible way to visualize recurrent brain tumors in pediatric patients and evaluate changes in metabolic tumor volume after one cycle (four weeks) or more of bevacizumab therapy.
Amino acid PET is a well-established technique for brain tumor imaging in adults and children. It has been used to augment MRI for surgical planning because the tracer frequently shows tumors that have extended beyond their boundaries. It can be used to monitor therapy, guide biopsies, and detect residual tumors and suspected recurrence.
Bevacizumab (Avastin, Genentech) is an antiangiogenic monoclonal antibody that binds to vascular endothelial growth factor (VEGF), but its role in neuro-oncology remains controversial. Additionally, MRI alone has limited ability to differentiate responders to bevacizumab from nonresponders, particularly early in the course of therapy.
"We wanted to see if we could use FDOPA-PET/MRI in children with recurrent gliomas [to assess] the efficacy of the anti-VEGF antibody bevacizumab," lead author Dr. Jonathan McConathy, PhD, an assistant professor of radiology in the division of nuclear medicine, told SNMMI attendees. "It is somewhat controversial whether bevacizumab is effective, as there is conflicting data. It is quite clear that MRI alone does not predict response to bevacizumab very well, particularly early after the start of therapy."
Related research
McConathy said this research on pediatric patients was influenced by a 2014 study from the University of California, Los Angeles (UCLA) on a group of adult patients with recurrent high-grade glioma (Clinical Cancer Research, July 1, 2014, Vol. 20:13, pp. 3550-3559).
The UCLA study found that a 35% decrease in metabolic tumor volume two weeks after starting bevacizumab was a predictor of overall survival. Based on FDOPA-PET scans, 17 patients who responded positively to bevacizumab survived 3.5 times longer (12.1 months) than 11 patients who did not respond well to bevacizumab (3.5 months). Based on MRI data, 22 responders to bevacizumab lived 1.5 times longer (11.4 months) than seven nonresponders (7.7 months).
In the current study, McConathy and colleagues enrolled six patients between the ages of 8 and 12 years who had relapsed brain tumors and were scheduled for treatment with bevacizumab. Patients received a baseline PET/MRI scan (Biograph mMR, Siemens Healthcare), which was followed by one cycle (four weeks) of bevacizumab, a follow-up FDOPA-PET/MRI, and then another cycle of bevacizumab.
The goal was to establish whether FDOPA-PET/MRI is safe in the pediatric population; that the protocol could acquire at least 45 minutes of PET/MRI data; and whether absolute changes in standardized uptake values (SUVs) and metabolic tumor volume could be measured.
Pediatric patient issues
"One reason we are very interested in using simultaneous PET/MRI in this patient population is because we can perform dynamic PET as well as a comprehensive MRI tumor protocol in one session," McConathy said. "That is particularly appealing for children, so we don't have to bring them back twice [to repeat the same scan]. We did not use sedation in our studies, but the potential for doing everything in one sedation or anesthesia session is very appealing for children."
The subjects were asked to fast for four hours prior to imaging. The dose of FDOPA was based on the patent's weight (0.08 mCi/kg) and ranged from 2 mCi to 5 mCi. Dynamic patient data were acquired 45 to 60 minutes after injection, and a standard pre- and postcontrast brain tumor protocol was used for the MRI portion of the scan, which took an average of 35 minutes.
The researchers manually drew regions of interest over the tumors and the left frontal cortex, which was used as the normal part of the brain for FDOPA and PET and MR images. Thresholds for metabolic tumor volumes were defined based on the mean SUV of the normal brain reference region.
The FDOPA-PET/MRI scans showed a wide range of initial tumor volumes among the six pediatric patients, ranging from 1.1 mL to 52.5 mL. After bevacizumab, FDOPA-PET/MRI showed a dramatic decrease in tumor size, with the percentage of tumor shrinkage ranging from 31% (52.4 mL to 36.2 mL) to 99% (8.4 mL to 0.04 mL).
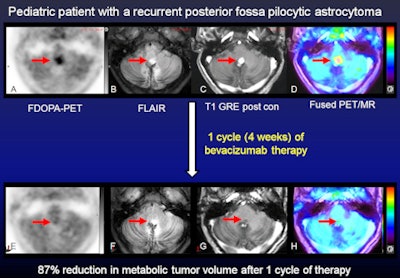 Dynamic FDOPA-PET and multiplanar pre- and postcontrast MR images were acquired simultaneously before and after bevacizumab. Representative axial images at baseline (A-D, top row) and after one cycle (four weeks) of bevacizumab (E-H, bottom row) are shown. There is focally increased FDOPA uptake in the tumor (A) with FLAIR hyperintensity (B) and dense postcontrast enhancement (C) (red arrows). The FDOPA-PET images demonstrate an 87% decrease in metabolic tumor volume after bevacizumab based on the amount of residual uptake 1.5-fold or greater than the mean SUV of the normal left frontal lobe reference region. In contrast, FLAIR MRI (G) shows no substantial change in tumor size, while postcontrast MRI (H) shows decreased contrast enhancement but no decrease in tumor size as expected with bevacizumab. Follow-up brain MRI for this patient over six to 12 months showed a decrease in tumor size from bevacizumab therapy. Images courtesy of Dr. Jonathan McConathy.
Dynamic FDOPA-PET and multiplanar pre- and postcontrast MR images were acquired simultaneously before and after bevacizumab. Representative axial images at baseline (A-D, top row) and after one cycle (four weeks) of bevacizumab (E-H, bottom row) are shown. There is focally increased FDOPA uptake in the tumor (A) with FLAIR hyperintensity (B) and dense postcontrast enhancement (C) (red arrows). The FDOPA-PET images demonstrate an 87% decrease in metabolic tumor volume after bevacizumab based on the amount of residual uptake 1.5-fold or greater than the mean SUV of the normal left frontal lobe reference region. In contrast, FLAIR MRI (G) shows no substantial change in tumor size, while postcontrast MRI (H) shows decreased contrast enhancement but no decrease in tumor size as expected with bevacizumab. Follow-up brain MRI for this patient over six to 12 months showed a decrease in tumor size from bevacizumab therapy. Images courtesy of Dr. Jonathan McConathy.Based on the results, McConathy and colleagues concluded that FDOPA-PET was tolerated well by the six patients, who underwent a total of nine PET/MRI scans and one PET/CT study.
"The main technical challenge we have had in the nonsedated children is that later in the scan they will move their heads," he added. "A lot of these children will fall asleep during the scan, wake up, and move slightly. This can be corrected, but it is a potential issue."
The group plans to continue the research in a larger study of pediatric patients to validate the results and to see if FDOPA-PET/MRI can be expanded to other pediatric applications, such as neuro-oncology.
"One question in PET/MRI is: How good is your brain attenuation?" McConathy said. "We also want to compare CT and MRI in attenuation correction to see if this affects our quantitation."
Study disclosures
McConathy receives research support from Eli Lilly/Avid Radiopharmaceuticals and GSK and is a consultant for Siemens and Blue Earth Diagnostics.