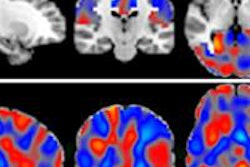
Should visual or automated analysis be used with PET imaging to predict a person's progression from mild cognitive impairment to dementia due to Alzheimer's, based on a two-year follow-up evaluation? It could depend on the radiotracer involved.
A new study from German researchers, published in the February issue of the Journal of Nuclear Medicine, concluded that the techniques were equally effective with carbon-11-labeled Pittsburgh Compound B (C-11 PiB) for ruling out Alzheimer's disease, with negative predictive values (NPVs) of 100%. Meanwhile, with FDG-PET, visual analysis was preferred over an automated software technique.
On the downside, both FDG-PET and C-11 PiB had relatively low positive predictive values (PPVs), which "could make it challenging to predict the clinical course of the disease," the authors wrote.
2 options for diagnosis
Both C-11 PiB and FDG have been used successfully to evaluate the potential risk of patients advancing from mild cognitive impairment to dementia and Alzheimer's disease.
C-11 PiB PET has primarily been used to identify or rule out the conditions, while FDG-PET has proved beneficial in assessing the location and severity of neuronal dysfunction and in predicting whether mild cognitive impairment could lead to dementia.
In addition, there has been some debate over whether results from these two imaging techniques should be evaluated using visual or automated means.
"There is currently no consensus as to whether amyloid and FDG-PET images should be analyzed visually or via fully automated procedures and which of these methods is better at determining whether a patient with mild cognitive impairment is likely to progress to dementia," wrote lead author Dr. Timo Grimmer, from Technische Universität München, and colleagues (JNM, February 2016, Vol. 57:2, pp. 204-207).
Analysis tools
The researchers enrolled 28 patients (14 men and 14 women) with an average age of 67.1 years (± 7.3 years) at the time of the first baseline evaluation for cognitive impairment; subjects already had a Clinical Dementia Rating (CDR) score of 0.5, indicating the presence of mild cognitive impairment. A CDR score of 0 is considered normal with no dementia, 0.5 indicates mild cognitive impairment, and greater than 0.5 is classified as dementia.
The cohort was evaluated again at least two years later, with a mean follow-up time of 31.2 months (± 7.8 months). During that time, nine patients developed dementia due to Alzheimer's, two people developed frontotemporal dementia, and one individual progressed to moderate dementia of unknown cause. The patients were then scanned with C-11 PiB PET and FDG-PET, following standard protocols.
The visual analysis scores were based on the interpretations of two independent, board-certified nuclear medicine specialists who were blinded to all clinical information and automated analysis results.
The automated analysis scores were based on calculations made by two software-based techniques. For C-11 PiB analysis, the researchers used a tissue model that calculates the presence of amyloid using the cerebrum-to-vermis ratio (vermis being the area between both hemispheres of the cerebellum, which is associated with movement).
For FDG images, Grimmer and colleagues used PMOD Technologies' Alzheimer's discrimination analysis software, which is designed to categorize results as either normal or abnormal based on FDG uptake in regions of the brain known to harbor Alzheimer's.
Rating the techniques
The automated and visual analysis techniques used to interpret C-11 PiB PET images both scored a negative predictive value of 100% in determining the progression of mild cognitive impairment to dementia due to Alzheimer's. This was higher than the use of either technique with FDG-PET, at 65% NPV for the automated technique and 78% for visual analysis.
| PET analysis results by radiotracer | |||||
| PPV | NPV | Accuracy | |||
| C-11 PiB | |||||
| Visual analysis | 50% | 100% | 68% | ||
| Automated analysis | 53% | 100% | 71% | ||
| FDG | |||||
| Visual analysis | 32% | 65% | 68% | ||
| Automated analysis | 37% | 78% | 50% | ||
Positive predictive values achieved by C-11 PiB and FDG were not very impressive in determining the potential progression of mild cognitive impairment, regardless of visual or automated analysis. C-11 PiB was only "slightly superior" to FDG at the two-year follow-up, the authors wrote.
"This suggests that neither amyloid nor FDG assessments lend themselves to predicting a conversion from mild cognitive impairment to dementia due to Alzheimer's within a short follow-up interval of two years," they wrote. "Therefore, physicians might be well-advised to exert caution with regard to the type of information provided to patients based on neuroimaging findings at the time of imaging."
Grimmer and colleagues also suggested that the low PPVs may be because patients "with identical pathologies may vary greatly with regard to factors that determine their resilience to Alzheimer's." As a result, it "could make it challenging to predict the clinical course of the disease."
Accuracy rates for FDG-PET were greater with the visual analysis (54%-68%) than the automated analysis (20%-50%), prompting the researchers to favor visual analysis as the better option for FDG-PET in these cases.
Accuracy with C-11 PiB was similar between the automated analysis (71%) and the visual analysis (68%).
Grimmer and colleagues cited the small sample size as a major study limitation, adding that the "findings should be confirmed in a larger group of patients," although PPV and NPV results using the automated analysis with FDG-PET are "comparable to a previous study that included a larger cohort."