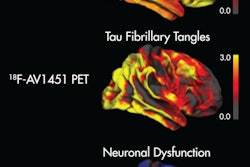
Japanese researchers are reporting early success with a novel PET imaging tracer that enhances the detection of tau deposits in regions of the brain that are associated with the onset of cognitive impairment and Alzheimer's disease.
The tracer, known as F-18 THK5351, demonstrated a greater ability to selectively bind to tau accumulations in certain brain regions that are prime targets for neurodegeneration and dementia. The findings were published in the February issue of the Journal of Nuclear Medicine.
Interestingly, in certain comparisons, THK5351 outperformed another PET tracer, F-18 THK5117, which received the Image of the Year award at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2014 annual meeting.
As lead author Dr. Ryuichi Harada and colleagues from Tohoku University School of Medicine in Sendai noted, the accumulation of tau protein in the brain has been considered a catalyst for neurodegeneration. PET is considered essential to gathering information on the progression of tau pathology in the living brain.
The next critical step, then, is to develop a potent PET tracer that can highlight the presence of tau protein.
Harada and colleagues authored a 2015 study that evaluated two PET tracers -- THK5105 and THK5117 -- and found they both demonstrated increased uptake in common sites of tau pathology in patients with Alzheimer's and dementia (European Journal of Nuclear Medicine and Molecular Imaging, July 2, 2015).
"However, these tracers -- like amyloid PET tracers -- showed high nonspecific retention in subcortical white matter," the authors wrote in the current study. "This white-matter binding must be minimized because the signals could obscure visual interpretation of PET images and decrease detection sensitivity for early tau pathology in the presymptomatic stage of Alzheimer's disease."
To resolve that issue, the researchers replaced a benzene ring of THK5117 with pyridine, creating THK5351.
Tracer protocols
To test the efficacy of this new agent, Harada and colleagues performed THK5351-PET scans on three Alzheimer's patients and three healthy elderly subjects. Two of the Alzheimer's subjects also underwent additional THK5117-PET scans within two-week intervals and additional PET exams using carbon-11-labeled Pittsburgh Compound B (C-11 PiB) within three-month intervals (JNM, February 2016, Vol. 57:2, pp. 208-214).
PET imaging with C-11 PiB has shown success in detecting beta-amyloid deposits, which have been associated with the onset of dementia in older adults.
Dynamic PET images (Eminence Stargate, Shimadzu Medical Systems) were acquired 90 minutes after intravenous injection of THK5117 (185 MBq) or THK5351 (185 MBq) and 70 minutes after administration of C-11 PiB (296 MBq).
The researchers also calculated standardized uptake values (SUVs) for the images from each of the three tracers by normalizing tissue radioactivity concentration by injected dose and body weight.
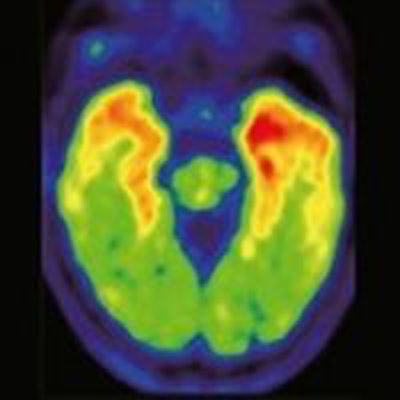
 THK5351-PET scans 40 to 60 minutes after injection show retention in the temporal lobe, which distinguishes Alzheimer's disease (AD) patients from healthy elderly participants (HC). Image courtesy of JNM.
THK5351-PET scans 40 to 60 minutes after injection show retention in the temporal lobe, which distinguishes Alzheimer's disease (AD) patients from healthy elderly participants (HC). Image courtesy of JNM.Harada and colleagues found that THK5351 retention in the temporal lobe (SUV range, 1.45-2.36) "clearly distinguished" Alzheimer's patients from healthy elderly participants (SUV range, 1.36-1.67), although mild THK5351 retention was observed in the medial temporal cortex of the healthy control subjects.
Among the Alzheimer's patients, THK5351's ability to bind in the inferior temporal cortex was greater than its binding to white matter at all time points of the PET scan after injection, thus providing enhanced detection of tau deposits.
While the peak SUV of THK5351 was slightly lower than the SUV for THK5117, the authors also noted that THK5351 cleared more rapidly than THK5117 from the cerebellar cortex. They also found no significant retention of THK5351 in the choroid plexus or venous sinus regions.
"This is one of the advantages over the other tau tracers, because off-target retention in these areas might cause a spill-in of the tracer signals into the brain," the authors wrote.
More importantly, the cortical-to-white-matter ratio of THK5351 in Alzheimer's patients was "substantially higher than that of THK5117, which allows comparatively easier and more accurate visual interpretation of PET images," they wrote.
THK5351's high contrast and low white-matter retention in this first-in-human PET study of Alzheimer's patients allows for the "sensitive detection" of tau pathology and could help in the early detection and assessment of neurodegeneration, Harada and colleagues concluded.