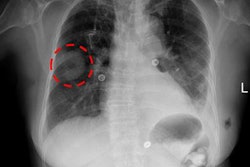
The U.S. National Institutes of Health (NIH) has suspended work at two facilities -- one of them producing PET materials -- due to problems with quality and safety standards.
NIH said that production was suspended at a PET facility within the National Institute of Mental Health, as well as at a lab engaged in cell therapy production in the National Cancer Institute. There is no evidence that patients have been harmed, but NIH said it would not enroll individuals in any new clinical trials until the issues are resolved.
The suspensions were the outcome of an evaluation performed by two companies that NIH hired to investigate quality assurance for manufacturing and compounding at all of its facilities that produce sterile or infused products for administration to research participants. NIH commissioned the investigation after serious problems were identified last year in the agency's Clinical Center Pharmaceutical Development Section.