PET/CT with the radiopharmaceutical gallium-68 (Ga-68) DOTATATE is a viable option to indium-111 (In-111) pentetreotide scans to detect neuroendocrine tumors, according to a study published in the May issue of the Journal of Nuclear Medicine.
Researchers also concluded that Ga-68 DOTATATE PET/CT could greatly advance treatment management for these patients.
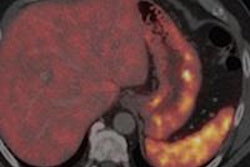
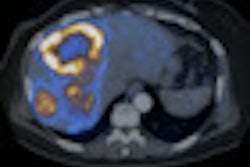
The study compared both imaging methods on 78 of 97 consecutively enrolled patients with known or suspected pulmonary or gastroenteropancreatic neuroendocrine tumors. The researchers found that Ga-68 DOTATATE PET/CT combined with CT and/or liver MRI changed care in 28 (36%) of the 78 patients (JNM, May 2016, Vol. 57:5, pp. 708-714).
Ga-68 DOTATATE PET/CT also correctly identified three patients for peptide receptor radiotherapy who had been incorrectly classified by In-111 pentetreotide.
Study co-author Dr. Ronald C. Walker from Vanderbilt University and colleagues also listed the benefits of Ga-68 DOTATATE, noting no significant toxicity, lower radiation exposure, and improved accuracy of results.
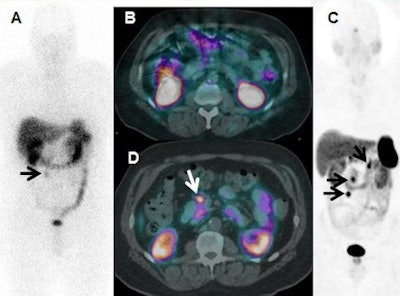
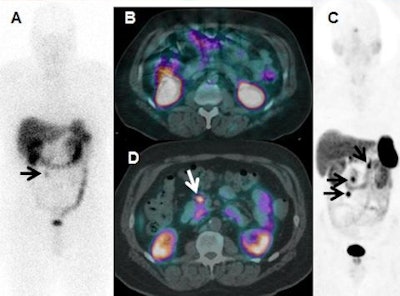 In-111-DTPA-octreotide scan (A) demonstrates recurrent mesenteric nodule (arrow) on the anterior view in a patient with a midgut carcinoid tumor resected five years previously, with no other tumor seen with planar imaging or SPECT/CT (B). Ga-68 DOTATATE PET/CT anterior 3D maximum intensity projection (C) and axial fused images (D) show other metastases (arrows), changing the surgical plan for resection. Findings were verified at surgery. Image courtesy of Dr. Ronald C. Walker.
In-111-DTPA-octreotide scan (A) demonstrates recurrent mesenteric nodule (arrow) on the anterior view in a patient with a midgut carcinoid tumor resected five years previously, with no other tumor seen with planar imaging or SPECT/CT (B). Ga-68 DOTATATE PET/CT anterior 3D maximum intensity projection (C) and axial fused images (D) show other metastases (arrows), changing the surgical plan for resection. Findings were verified at surgery. Image courtesy of Dr. Ronald C. Walker.On the downside, the U.S. Food and Drug Administration (FDA) has yet to approve Ga-68 DOTATATE PET/CT to diagnose, stage, or help determine treatment for patients with neuroendocrine tumors. As a result, there is no reimbursement for the procedure.