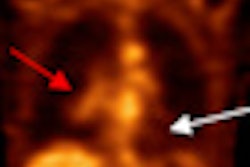
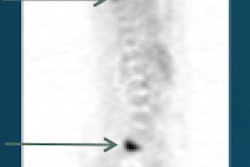
Biotechnology firm Cell>Point has inked a licensing deal with U.S. and China consortium United Eastern Pharmaceutical (UEP) for its Oncardia radiopharmaceutical (ethylenedicysteine-glucosamine) in China, Hong Kong, and Macau.
Cell>Point's exclusive license with UEP provides for upfront and regulatory milestone payments as well as royalties on sales of Oncardia, which is based on an ethylenedicysteine drug conjugate platform licensed by Cell>Point from MD Anderson Cancer Center. Oncardia was designed for labeling with technetium-99m for SPECT or gallium-68 for PET in imaging cancer and cardiovascular disease, according to the vendor.
Cell>Point said it will seek regulatory approval from the China Food and Drug Administration to expand its Oncardia phase III lung cancer imaging trial to sites in China. In addition, UEP said it plans to pursue additional approvals in China for the diagnosis and staging of lung, head and neck, colorectal, breast, liver, stomach, lymphoma, and other cancers, along with the diagnosis of cardiac ischemia.