Researchers from Yale University are reporting promising results with a PET tracer designed to assess synaptic density in patients with Alzheimer's disease. The hope is that the approach could become an in vivo biomarker for dementia and help in therapy development, according to a paper published online July 16 in JAMA Neurology.
This study builds upon previous work by the same researchers, who found that the carbon-11 (C-11) UCB-J tracer has "excellent characteristics" for quantitative imaging in vivo in both nonhuman primates and humans, targeting a particular protein that is expressed in nearly all synapses.
"Until now, the assessment of synaptic density and the quantification of presynaptic proteins in Alzheimer's disease could only be performed in postmortem brain tissues," wrote lead author Dr. Ming-Kai Chen, PhD, and colleagues. "The present study demonstrated, for the first time, that noninvasive PET imaging with C-11 UCB-J is capable of measuring reductions in synaptic density in vivo in the hippocampus of individuals with amnestic mild cognitive impairment and mild Alzheimer's dementia."
Alzheimer's disease is characterized by the accumulation of beta-amyloid plaques, neurofibrillary tangles, and synaptic loss. The synapses are crucial for cognitive function, and damage to them is often seen in early-stage Alzheimer's and mild cognitive impairment.
"Thus the ability to assess synaptic density in vivo would greatly enhance clinical research in Alzheimer's and would specifically provide a valuable biomarker outcome for therapeutic trials," the authors wrote. "However, F-18 FDG is not a direct biomarker of synaptic density, and test results may be confounded by sensory stimulation, medications, and blood glucose level."
For PET to be used in monitoring synaptic density, the key is to find effective molecular targets. One such target appears to be a protein known as synaptic vesicle glycoprotein 2 (SV2). One of its isoforms, SV2A, is expressed in virtually all synapses and potentially could be an indicator of synaptic density in Alzheimer's and other neuropsychiatric disorders, Chen and colleagues wrote.
The study included 10 participants with Alzheimer's disease and 11 cognitively normal individuals who were age-, gender-, and education-matched to the Alzheimer's group. Five of those in the Alzheimer's group had amnestic mild cognitive impairment, while five had mild dementia. Mean age was 72.7 (± 6.3) years for the Alzheimer's group and 72.9 (± 8.7) years for the cognitively normal group (JAMA Neurol, July 16, 2018).
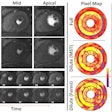
All participants received one injection of C-11 UCB-J (493 ± 224 MBq), with no significant difference in radioactivity or mass dose between groups. They also underwent high-resolution PET scans with C-11-labeled Pittsburgh Compound B (PiB) to detect beta-amyloid plaque, along with MRI scans and cognitive and neurologic evaluations. Results were based on C-11 UCB-J specifically binding to SV2A in the hippocampus, where neurodegeneration would be most prominent.
"We hypothesized a reduction in hippocampal SV2A binding in Alzheimer's, based on the early degeneration of entorhinal cortical cell projections to the hippocampus and hippocampal SV2A reductions that had been observed in postmortem studies," the authors wrote.
Their premise proved accurate. PET images showed that subjects with Alzheimer's disease had a significant reduction in hippocampal SV2A binding (41%), compared with cognitively normal participants. The reductions in binding remained significant even after the researchers corrected for brain atrophy.
"This method provides a direct measure of SV2A and synaptic density and therefore holds promise as a novel in vivo biomarker for Alzheimer's disease and an outcome measure for trials of disease-modifying therapies, particularly those that target the preservation and restoration of synapses," Chen and colleagues concluded.