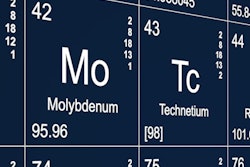
A nuclear reactor in South Africa has received government approval to resume production of medical radioisotopes, including molybdenum-99 (Mo-99). The move will ease a brief constriction in global supplies of radiopharmaceuticals.
NTP Radioisotopes received approval from South Africa's National Nuclear Regulator to resume production of medical radioisotopes at its Pelindaba facility. The reactor will start with preliminary production runs and then begin normal full production.
NTP's processing facility had been shut down since November 2017 after a series of safety-related issues. At the time, the Pelindaba facility was producing as much as one-third of the world's supply of molybdenum, which is the precursor to technetium-99m, a widely used radioisotope for nuclear cardiology studies.
In announcing the resumption, an NTP press release said that while the National Nuclear Regulator's processes "were sometimes seen as time-consuming," they were essential in ensuring that the facility was completely ready to resume operation.
"The nuclear regulator's primary mandate is to ensure that all safety standards are met, so that we can achieve safe operating conditions. The uncompromised integrity and independence of their work is what protects us all," said South Africa Deputy Minister of Energy Thembisile Majola in the statement.
After a brief period of tight supplies, global availability of molybdenum is expected to improve as nuclear reactors in the Netherlands and Australia return to normal production, according to an announcement issued November 13 by the Society of Nuclear Medicine and Molecular Imaging (SNMMI).