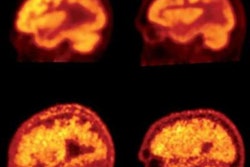
Clinicians may soon have a new PET tracer to improve the detection of tau protein tangles in the brain, advancing the diagnosis of Alzheimer's disease and subsequently leading to more effective treatments for dementia, according to a study published in the December issue of the Journal of Nuclear Medicine.
The radioligand is known as F-18 RO-948. So far, it has shown "good brain uptake, has no apparent brain-penetrant radiolabeled metabolites, has a good kinetic profile, [and] shows little or no retention in cognitively normal [controls]," the researchers wrote (JNM, December 2018, Vol. 59:12, pp. 1869-1876).
"Importantly, this new tracer appears to have much less off-target binding than was reported for existing tau tracers," added lead author Dr. Dean Wong, PhD, from Johns Hopkins University, in a statement from the Society of Nuclear Medicine and Molecular Imaging (SNMMI). "Especially, it has less binding to the choroid plexus adjacent to the hippocampus, which has confounded interpretation of mesial temporal tau measured by first-generation PET tau tracers."
The current study follows related preclinical research published in the April 2018 issue of JNM. In that study by Honer et al, three tau-specific radiopharmaceuticals -- carbon-11 (C-11) RO-963, C-11 RO-643, and F-18 RO-948 -- showed promise for in vitro and in vivo imaging of tau pathology in humans.
Wong and colleagues took those three tracers for their human trial and performed PET scans in three different groups:
- Alzheimer's patients (≥ 50 years) who were amyloid-PET positive
- A group of younger control subjects (25-40 years)
- A group of older control subjects (≥ 50 years)
Four Alzheimer's subjects underwent a second F-18 RO-948 PET scan between six and 22 months after the first exam. In addition, six healthy controls underwent a whole-body PET scan with F-18 RO-948.
Wong and colleagues found that peak standardized uptake values (SUVpeak) were highest with F-18 RO-948 across all brain regions and among all subjects. In light of that trend, they performed a voxel-wise and region-based analysis of F-18 RO-948; it showed multiple areas with statistically significant differences in tau binding between Alzheimer's patients and healthy controls. The voxel-wise analysis also identified a set of symmetric clusters where Alzheimer's patients had statistically significant greater binding than healthy controls (p < 0.001, cluster size > 50).
An analysis of variance (ANOVA) among 22 high tau-binding regions further revealed 13 areas (60%) with statistically significant differences in tau binding between the two groups (p < 10-5). In short, F-18 RO-948 outperformed the other two prospective tau tracers in the study and compared "favorably with other existing tau PET tracers," the researchers wrote.
"It is our hope that tools such as F-18 RO-948 will allow us to gain a better understanding of the pathophysiology of Alzheimer's disease and, in the context of drug development, select patients for clinical trials, confirm the mechanism of action of drugs targeting pathologic tau, and monitor the effects of disease-modifying therapies regardless of whether they target tau directly," Wong and colleagues concluded.