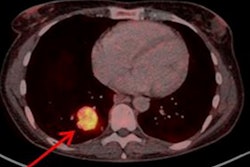
A new radiopharmaceutical based on lutetium-177 (Lu-177) being tested for the treatment of neuroendocrine tumors (NETs) is safe and effective and may allow physicians to cut the radiation dose patients receive by half, according to a study published online March 19 in the Journal of Nuclear Medicine.
Researchers led by Xiaoyuan Chen, PhD, of the National University of Singapore found that a new radiopharmaceutical, Lu-177 DOTA-EB-TATE (EBTATE, Molecular Targeting Technologies), was well tolerated and as effective as current standard treatment with Lu-177 DOTATATE (Lutathera, Advanced Accelerator Applications), but it required significantly less dosage.
The difference? That "-EB-" in the new drug's name.
NETs are rare tumors that originate in the neuroendocrine cells of numerous organs, and they are most commonly found in the lungs, gastrointestinal tract, and pancreas. The incidence of NETs reportedly increased 6.4-fold from 1973 to 2012. However, because they are rare, varied, and slow growing, the diagnosis of a neuroendocrine tumor can be delayed up to seven years. As a result, more than 50% of NETs are at an advanced stage at the time of diagnosis.
The current standard of care for patients with late-stage neuroendocrine tumors is peptide receptor radionuclide therapy (PRRT) with Lu-177 DOTATATE, which is flushed from a patient's system rapidly after administration. Preclinical studies found that if a special dye (Evans blue, or EB) is added to Lu-177 DOTATATE, the treatment lasts longer and is more effective. EB binds reversibly to serum albumin to enhance the radiotherapeutic time window with longer circulation half-life and increase tumor uptake/retention to improve the therapeutic outcome.
In this open-label, dose-escalation study, the researchers sought to determine what dose of the modified therapy is safest and produces the best tumor response compared to the current recommendation of 7.4 GBq (200 mCi) every eight weeks for a total of four doses. They enrolled 32 NET patients and randomly divided them into three groups. Group A received 1.17 ± 0.09 GBq/cycle, group B received 1.89 ± 0.53 GBq/cycle, and group C received 3.97 ± 0.84 GBq/cycle. The treatment was planned for up to three cycles.
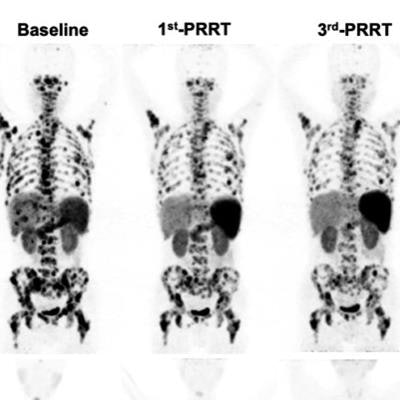
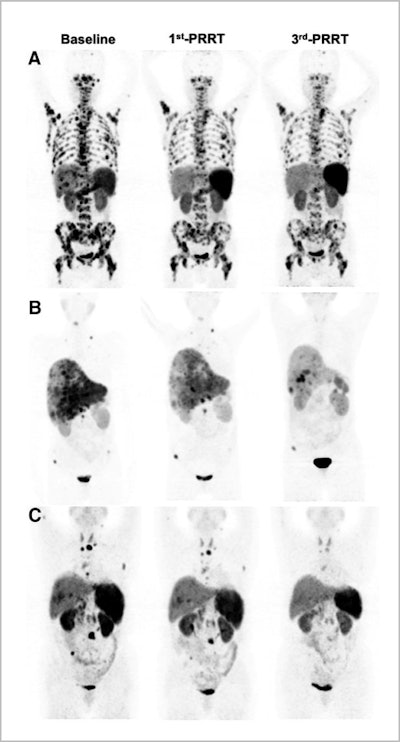 Representative images of partial remission in group A (A), group B (B), and group C (C). Image created by Qingxing Liu and Zhaohui Zhu/Peking Union Medical College Hospital, Beijing, and courtesy of JNM.
Representative images of partial remission in group A (A), group B (B), and group C (C). Image created by Qingxing Liu and Zhaohui Zhu/Peking Union Medical College Hospital, Beijing, and courtesy of JNM.Overall disease control rates were higher in groups B (83.3%) and C (71.5%) than in group A (66.7%). Ultimately, the researchers found that the Lu-177 DOTA-EB-TATE dose of 1.89 GBq/cycle was the most effective for tumor control in NET patients. They also noted that with careful patient selection and monitoring, a 3.97-GBq/cycle dose could achieve an even better response.
Generally, patients tolerated the lower doses well, with no immediate common adverse effects, such as irritating pain, allergy, or fever during administration, and no life-threatening adverse effects during the observation period. One patient in group C experienced nausea and vomiting for several hours after treatment but recovered within two weeks. Common Terminology Criteria for Adverse Events grade 3 hepatotoxicity (elevated aspartate aminotransferase) was recorded in one patient in group A and one patient in group C. No nephrotoxicity was observed.
"In terms of NETs, the more effectively we can use 177Lu for treatment, the more we will be able to reduce the financial strain on patients," said Chen in a statement from the Society of Nuclear Medicine and Molecular Imaging. "Overall, this new treatment has the potential to significantly impact mortality and morbidity for neuroendocrine tumor patients."