PET imaging with a carbon-11 (C-11) glutamine radiotracer appears to be a safe and effective method for detecting metastatic colorectal cancer, according to a first-in-human study of the radiotracer published April 30 in the Journal of Nuclear Medicine.
Researchers observed no adverse or clinically detectable pharmacologic effects after injecting metastatic colorectal cancer patients with C-11 glutamine. They did observe that uptake of C-11 glutamine was elevated and visualized in lung, brain, bone, and liver metastases, which suggests the radiotracer could serve as a new tool for therapies targeting glutamine metabolism in metastatic colorectal cancer.
"Imaging with [C-11] glutamine can complement other imaging approaches, including [F-18] FDG PET and PET imaging with other tracers currently in development, thus providing a more complete picture of the patient's disease and underlying biological processes in individual metastatic sites," wrote Allison Cohen, PhD, lead author and staff scientist at the University of Texas MD Anderson Cancer Center, and colleagues.
C-11 glutamine was synthesized several years ago and is chemically and biologically identical to physiological glutamine, a key nutrient for normal cellular growth and proliferation. Cells that avidly take up glutamine will also avidly take up C-11 glutamine. In cancer, tumor cells hijack the process to fuel tumor growth, which suggests C-11 glutamate may be an effective marker for understanding and perhaps blocking the process at a molecular level. C-11 glutamine has been studied in preclinical mouse models but not to date in humans.
In this study, researchers primarily sought to evaluate the radiologic safety and biodistribution of C-11 glutamine for cancer PET imaging. They enrolled nine patients in a clinical trial at Vanderbilt University Medical Center. All patients were adults with metastatic wildtype KRAS colorectal cancer who had received prior anti-epidermal growth factor receptor therapy.
Patients underwent baseline evaluation of disease by CT or MRI and had at least one measurable lesion. Dynamic PET scans centered over the abdomen or thorax were performed at the same time as intravenous tracer administration. Patients received 337.97 ± 44.08 MBq of C-11 glutamine. Following the dynamic acquisition, a whole-body PET/CT scan was acquired.
C-11 glutamine was well-tolerated in all patients with no observed safety concerns. Organs with the highest radiation exposure included the bladder, pancreas, and liver. The estimated effective dose was 4.46E-03 ± 7.67E-04 mSv/MBq. Accumulation of C-11 glutamine was elevated and visualized in lung, brain, bone, and liver metastases, suggesting its utility for cancer imaging.
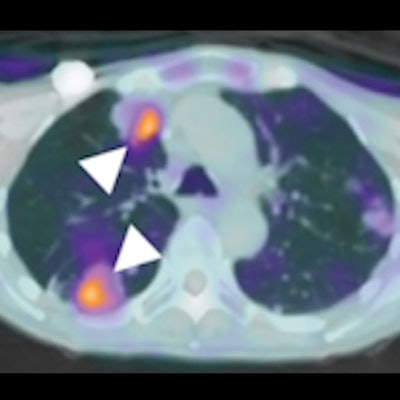
 C-11 glutamine tumor uptake in a patient with metastatic colorectal cancer. Axial C-11 glutamine PET/CT fusion images corresponding to a left lung metastasis (A), two right lung metastases (B), and a brain metastasis (C). White arrowheads point to the lesions. The lesion-to-blood pool ratios from the whole-body scan were 2.64 (A), 2.22 (B, top arrow), 2.35 (B, bottom arrow), and 1.68 (C). (D) A contrast-enhanced MRI was obtained 6.5 weeks after baseline PET imaging and treatment. The lesion is indicated with a white arrowhead. The MRI confirms the presence of the brain lesion seen with C-11 glutamine PET. Image courtesy of the Journal of Nuclear Medicine.
C-11 glutamine tumor uptake in a patient with metastatic colorectal cancer. Axial C-11 glutamine PET/CT fusion images corresponding to a left lung metastasis (A), two right lung metastases (B), and a brain metastasis (C). White arrowheads point to the lesions. The lesion-to-blood pool ratios from the whole-body scan were 2.64 (A), 2.22 (B, top arrow), 2.35 (B, bottom arrow), and 1.68 (C). (D) A contrast-enhanced MRI was obtained 6.5 weeks after baseline PET imaging and treatment. The lesion is indicated with a white arrowhead. The MRI confirms the presence of the brain lesion seen with C-11 glutamine PET. Image courtesy of the Journal of Nuclear Medicine."This study evaluated the safety and biodistribution of [C-11] glutamine and analyzed its ability to visualize metastatic lesions in patients with colorectal cancer. [C-11] glutamine was well-tolerated and showed increased uptake in tumors relative to background," the researchers wrote.
Moreover, the dose in the study was approximately one-fourth the average effective dose (2.05E-02 ± 7.6E-03 mSv/MBq) found from a review of 144 publications for a range of F-18 labeled tracers, according to the authors.
However, not all lesions identified in the patients in this study were glutamine avid, the researchers noted, which points to the importance of using multiple orthogonal approaches to studying cancer. Imaging with C-11 glutamine can complement other imaging approaches, including F-18 FDG-PET and PET imaging with other tracers currently in development, they stated.
"PET using [C-11] glutamine appears safe for human use and allows noninvasive visualization of metastatic colon cancer lesions in multiple organs. Further studies are needed to elucidate its potential for other cancers and for monitoring response to treatment," the authors concluded.