PET imaging has moved to the forefront of molecular imaging of atherosclerosis, according to a study published May 7 in the Journal of Nuclear Medicine. The researchers examined the new radiotracers that are available and described their pros and cons.
Recent immunometabolic discoveries in atherosclerosis suggest metabolic imaging approaches using FDG and other PET radiotracers may improve the risk stratification of patients and early monitoring of disease progression or response to therapy, according to the authors.
"The versatility of PET to provide insights into various metabolic pathways along with the growing discoveries in immunometabolism ... have placed it at the forefront of molecular imaging of atherosclerosis," wrote co-authors Philip Zachary Mannes and Dr. Sina Tavakoli, PhD, of the University of Pittsburgh.
Over the past decade, recognition of the links between intracellular metabolism and immune-cell activation and its consequences in atherogenesis has grown. Yet, the majority of research in the area has been in cultured cells and extrapolation to the complex microenvironment of plaques has been limited.
In vivo metabolic imaging with PET radiotracers is ideal for addressing this gap and elucidating the clinical implications of immunometabolic alterations for diagnosis and management of patients, according to the authors.
In this article, the researchers described recent advances in understanding of immunometabolism of atherosclerosis with an emphasis on macrophages and reviewed promising metabolic imaging approaches using FDG and other PET radiotracers.
F-18 fluorodeoxyglucose (FDG)
Following initial retrospective reports of FDG uptake in atherosclerosis, several studies demonstrated higher FDG uptake in plaques that have features of vulnerability and in symptomatic patients compared with stable/asymptomatic lesions. Further investigations revealed that FDG uptake in plaque correlates with its inflammatory burden, particularly macrophage content, and can be reproducibly quantified.
Moreover, FDG uptake in large arteries has been shown to correlate with various cardiovascular risk factors and improve the prediction of future cardiovascular events, including stroke and myocardial infarction, beyond conventional risk stratification tools, such as Framingham risk score and severity of carotid artery stenosis, the authors wrote.
Advantages of FDG include the following:
- Readily available
- Most extensively validated radiotracer in preclinical and clinical studies
- Uptake correlates with overall inflammatory burden of plaques (e.g., macrophage content)
- Early detection of response to statins
Limitations: Targets a nearly ubiquitous metabolic process with limited specificity for individual cell type or phenotype
Hypoxia
Two major classes of hypoxia imaging agents, including nitroimidazole (e.g., F-18 fluoromisonidazole [FMISO] and F-18 HX4) and copper complexed with diacetyl-bis (N4-methylthiosemicarbazone) (Cu-ATSM) analogs, have been used to image atherosclerosis in small clinical studies. Localized HX4 uptake in symptomatic carotid plaques was shown to correlate with arterial wall thickness and FDG uptake, while FMISO uptake was also found to be higher in symptomatic carotid plaques in patients with a recent stroke, according to the authors.
F-18 fluoromisonidazole (FMISO)
Advantages:
- Higher uptake in symptomatic carotid plaques
- Positive correlation between F-18 FMISO and F-18 FDG and uptake
Limitations: Diffusion barrier limits uptake; lack of cell-specificity
HX4
Advantages:
- Positive correlation between HX4 and FDG
Limitations: Diffusion barrier limits uptake; lack of cell specificity
Cu-64 ATSM
Advantages:
- Uptake correlates with plaque hypoxia and macrophage content
- Higher hypoxic cell uptake and faster washout from normoxic tissues than F-18 FMISO
Limitations: Diffusion barrier limits uptake; lack of cell specificity
"Future work is required to determine if imaging hypoxia allows for prospective identification of plaques at risk of acute complications or response to medical interventions," the authors wrote.
Acetate
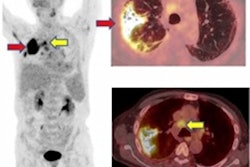
The feasibility of imaging C-acetate uptake by plaques along the large arteries has been shown in patients undergoing PET/CT for oncologic indications. High-resolution autoradiography of murine brachiocephalic arteries has also demonstrated that arterial uptake of acetate is primarily localized to macrophage-rich regions of plaques, the authors stated.
Advantages:
- Focal uptake in calcified plaques and arterial segments without calcifications
Limitations: Short half-life, limiting the availability
"Despite the promise of C-acetate, less attention has been given to plaque imaging using tracers derived from longer chain fatty acids," according to the authors.
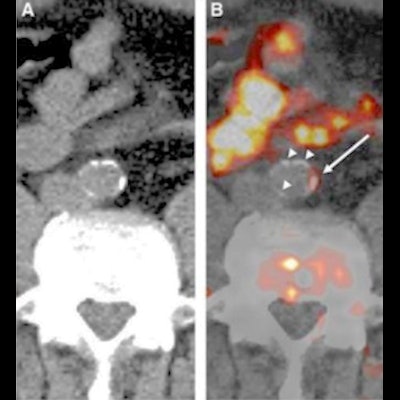
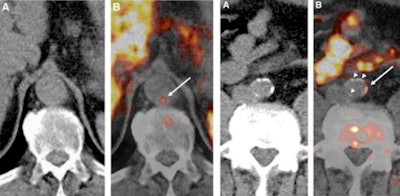 Examples of carbon-11 (C-11) acetate PET/CT of the aorta in patients receiving whole body scans for oncologic indications. Images from patient 1 (left panel, A and B) indicate C-11 acetate uptake in an aortic region without calcified plaques, whereas images from patient 2 (right panel, A and B) demonstrate colocalization of C-11 acetate uptake with aortic calcifications (white arrows point to areas of arterial C-11 acetate uptake).
Examples of carbon-11 (C-11) acetate PET/CT of the aorta in patients receiving whole body scans for oncologic indications. Images from patient 1 (left panel, A and B) indicate C-11 acetate uptake in an aortic region without calcified plaques, whereas images from patient 2 (right panel, A and B) demonstrate colocalization of C-11 acetate uptake with aortic calcifications (white arrows point to areas of arterial C-11 acetate uptake).Choline
Choline has emerged as a promising marker of cells with high rates of phospholipid biosynthesis, including proliferating cells. Multiple PET radiotracers, including C-choline, F-18 fluorocholine (FCH), and F-18 fluoroethylcholine (FEC) have been developed. The feasibility of choline-derived radiotracers for imaging calcified and noncalcified plaques within the aorta and iliac and carotid arteries has been shown in men undergoing PET imaging for prostate cancer.
F-18 fluoromethylcholine (FMC)
Advantages:
- Uptake is mostly in arterial segments with thickening and increased lipid content
Limitations: May be a substrate for a distinct set of transporters from those used to transport choline
F-18 fluoroethylcholine (FEC)
Advantages:
- Uptake correlates with cardiovascular risk factors
- No association between uptake and prior cerebrovascular or cardiovascular events
Limitations: May be a substrate for a distinct set of transporters from those used to transport choline
In addition, distinct patterns of FDG and C-glutamine uptake by aortic atherosclerotic plaques have been shown using high-resolution autoradiography in a mouse model of atherosclerosis, which suggests that combined imaging of glutamine and glucose metabolism may better delineate the immunometabolic heterogeneity of plaques.
Ultimately, a multidimensional approach extending from preclinical investigations to large-scale longitudinal clinical cohort studies and clinical trials is required to establish the role of immunometabolic imaging as a precision medicine tool in noninvasive plaque characterization, the authors stated.
"There is a dire need to transition towards in vivo immunometabolic phenotyping of atherosclerosis," they concluded.