Assessing brain tau pathology on PET scans over time on an individual rather than group level may provide a better way to test new treatments in Alzheimer's disease trials, according to a study published May 8 in JAMA Neurology.
Researchers led by Antoine Leuzy, PhD, of Lund University in Malmö, Sweden, found significantly higher estimates of annual change in tau on PET imaging using participant-specific brain regions of interest (ROIs) compared with the conventional group-level approach, where each participant is assigned the same ROI.
"Individualized ROIs carry an advantage over group-level ROIs for assessing longitudinal tau [pathology on] PET and can increase the sensitivity to detect treatment effects in Alzheimer's disease trials," the group wrote.
Tau neurofibrillary tangles that form in the brain are believed to drive the neurodegeneration associated with Alzheimer's disease, making longitudinal data from tau PET imaging pivotal in clinical trials for testing disease-modifying therapies. One key unanswered question when assessing potential treatment effects is whether the use of individual brain ROIs is superior to approaches where the same group-level ROI is used for each participant, the researchers explained.
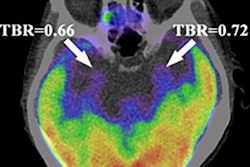
To address this issue, the group studied brain PET imaging of 406 patients enrolled in a prospective Alzheimer's disease clinical trial in Sweden between September 2017 and November 2021. The investigators included patients at different stages of the disease: cognitively unimpaired people, patients with mild cognitive impairment, and those with dementia. All study participants underwent PET imaging using tau-based radiotracers (F-18 RO948 or F-18 flortaucipir); they created standardized uptake value ratio images from the scans based on levels of radiotracer uptake in the brain. Mean follow-up time after baseline scans was 1.8 years.
Group-level ROIs included previously published whole-brain meta-ROIs and Braak stages used frequently in clinical trials to visually classify the severity of the disease, while individual ROIs were based on four tau PET subtypes similarly used to classify disease stages.
The investigators found percentage increases in tau PET across all ROIs. Group-level ROIs that showed the largest annual percentage increase in tau-PET standardized uptake value ratio in cognitively unimpaired individuals was seen in an ROI that combined the entorhinal cortex, hippocampus, and amygdala. In individuals with mild cognitive impairment, the greatest change was seen in the temporal cortical regions, whereas those with dementia had the greatest change in parietal regions.
However, "significantly higher estimates of annual change in tau PET were found using several of the participant-specific ROIs," the researchers reported.
Ultimately, the study contributes to the continuing investigation of optimized trial outcomes and precision medicine, the researchers noted.
"Future studies should assess additional methods for defining individualized ROIs," they concluded.