When AuntMinnie.com's article on nephrogenic systemic fibrosis (NSF) in patients with renal insufficiency ran in March 2007, medicolegal expert Dr. Leonard Berlin warned of the "malpractice pothole" that medical imaging professionals would find themselves trapped in should the number of NSF cases continue rack up.
A quick browse of the Internet indicates that some people are doing their best to make that pothole a little deeper and wider: for example, the Johnson Law Firm of Fort Worth, TX, has launched a Web site on NSF, urging patients to seek legal counsel to determine if they are eligible for "medical monitoring and compensation from the manufacturer (of the gadolinium contrast agent)."
Jumping on the NSF recompense bandwagon are other law firms, such as Ashcraft & Gerel of Washington, DC; Brian S. Campf of Portland, OR; and New York City-based Audet & Partners, all dangling free legal consultations in front of patients "and their loved ones" dealing with NSF.
Peter Brodhead, a partner with Cleveland-based Spangenberg, Shibley & Liber, is the plaintiff's attorney in the one pending NSF lawsuit (Walker vs. Tyco), in which attorneys for a 71-year-old dialysis patient in Cleveland filed suit against Mallinckrodt and its parent companies, Tyco Healthcare Group and Tyco International (now Covidien) of Pembroke, Bermuda, claiming that the patient developed NSF after a gadolinium-enhanced MRI scan (Optimark [gadoversetamide], Mallinckrodt, Hazelwood, MO) in 2006.
In Brodhead's opinion, NSF is merely a warning shot for the dangers that gadolinium poses in patients with renal insufficiency.
"(In) patients with renal insufficiency, there is the additional risk of nephrotoxicity," Brodhead told AuntMinnie.com. "While it was earlier thought that gadolinium-based contrast agents were less nephrotoxic than iodinated agents, recent studies have shown that when comparing equally attenuating doses of both agents, the gadolinium-based agents are far more nephrotoxic."
The obvious solution to this problem would be to swerve around the pothole and cease gadolinium use in all patients with renal insufficiency. But is that a realistic possibility? Yes, if MRI vendors have their way, as they hustle to provide new and/or improved noncontrast imaging protocols.
Putting the kibosh on contrast
Traditionally, renal disease has been an indication for MR angiography (MRA) rather than CT angiography (CTA) because of the possibility associated with nephrotoxicity of iodinated contrast. In certain types of MRA -- primarily body angiograms -- contrast is almost always necessary.
The concept of noncontrast MR is hardly a new one: MRA sans contrast media (and paired phase-contrast [PC] and time-of-flight [TOF] protocols), has been around for some time, as have noncontrast 2D turbo spin echo (TSE) and balanced steady-state free precession (SSFP).
But these protocols have drawbacks: They can be time-consuming and often yield poor-quality images, hampered by artifacts. More to the point, can noncontrast imaging do the same job as gadolinium-enhanced MR for imaging exams in patients with renal insufficiency?
Philips Medical Systems North America of Andover, MA, is working on new noncontrast techniques that include balanced fast field echo (bFFE), turbo spin echo (TSE), and fast scanning with new parallel imaging methods to improve PC acquisition.
The bFFE technique has been used successfully in multiple anatomy, as well as vessel wall imaging protocols using strategically placed SENSE coils, a proprietary technology Philips developed to reduce the amount of unneeded information collected in PC scans. It's available with cardiac-gated arterial/venous image acquisition.
 |
| Noncontrast-enhanced vascular imaging on a 1.5-tesla scanner (Achieva). Left, gated subtraction MRA. Top right, digital subtraction angiography. Bottom right, CT angiography. Images courtesy of Philips Medical Systems North America and Hyogo Brain and Heart Center, Hygogo, Japan. |
Joseph Fritz, Ph.D., Philips' director of MR clinical science, told AuntMinnie.com that Philips is actively researching many noncontrast-enhanced (non-CE) MR angles, because "noncontrast methods had been slow, and artifacts could yield false positives." Fritz said the new techniques include "dynamic blood velocity imaging with PC with KT (parallel imaging) SENSE, or very fast local field-of-view imaging with PC." Philips has launched a Web forum that offers tips on how to make the most of the company's protocols.
For Toshiba America Medical Systems of Tustin, CA, the widespread use of the contrast agent didn't stop it from continuing to explore noncontrast imaging techniques.
"Gadolinium wasn't being reimbursed in Japan, so Toshiba really made a push to develop noncontrast techniques that were better than time-of-flight," explained Mark Totina, product manager of Toshiba's MR business unit. One potential application is time spatial labeling inversion pulse (Time-SLIP).
"Time-SLIP without contrast is better than contrast-enhanced renal (MR)," Totina asserted. Gadolinium makes "the renal parenchyma light up and obscure the view of the vessel. In Time-SLIP, the renal parenchyma doesn't light up, so we see vessels that we've never seen before, even with contrast enhancement."
 |
| Above, high-resolution contrast-enhanced renal MRA with inadequate depiction of the segmental renal branches. Below, noncontrast Time-SLIP renal MRA depicting the main renal artery, as well as the segmental branches. Images courtesy of Toshiba America Medical Systems. |
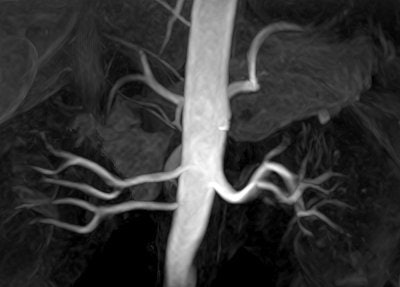 |
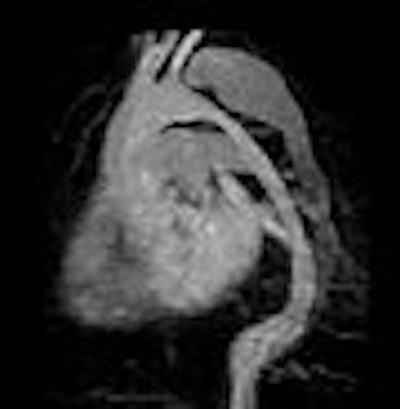
Meanwhile, at Siemens Medical Solutions of Malvern, PA, the focus is on angiography and perfusion. Dr. Milind Dhamankar, manager of clinical MR research collaborations, told AuntMinnie.com: "We are working on turbo spin echo, TrueFISP (balanced sequence), and HASTE (fast spin echo), essentially in the areas of carotid, renals, abdominal aortas, and peripheral vasculature." Cardiac gating and real-time variants are in the works, too.
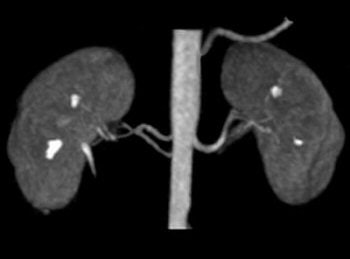 |
| Above, contrast-enhanced renal MRA on a Magnetom Avanto scanner. Imaging protocol included 3D FLASH with TR/TE 2.5/1/1 msec in 20 seconds. Below, 3D TrueFISP fat-saturated navigator-gated noncontrast renal MRA. Time: Five minutes. TR/TE 900/1.6 msec. Inversion time 600 msec (work-in-progress). Images courtesy of Siemens Medical Solutions USA. |
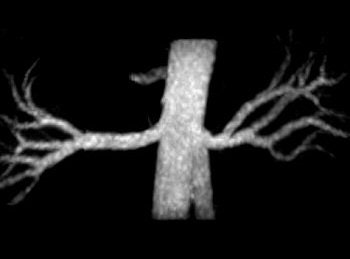 |
Also in the mix is noncontrast-enhanced MR tailored for oncologic perfusion and angiogenesis applications. "We're (testing) arterial spin labeling (ASL), which uses the intrinsic water in the blood as a tagging element," Dhamankar said. "We think it's going to be big for determining cerebral blood flow quantification."
The TSE, TrueFISP, HASTE, and ASL techniques are all in clinical trials in Europe, Japan, and the U.S. Siemens expects to bring ASL to market within a year, Dhamankar said.
Docs weigh in: Minimizing exposure
Clearly, any replacement protocol for gadolinium-based MRI has its work cut out for it. Dr. Harm Peters, a nephrologist at the Charite School of Medicine at Humboldt University in Berlin, questioned the validity of doing away with gadolinium altogether given that his specialty requires very specific information from an MR study.
"I want to have an overview of the soft-tissue organs, as well as the blood flow (arterial and venous vessels) and the urine flow, especially in the context of abdominal surgery and kidney transplantation," Peters told AuntMinnie.com. "Right now, I do not think that noncontrast MRI can satisfactorily answer the questions in renally insufficient patients. We need information on blood perfusion and urine flow. This is especially important for renally insufficient patients who have frequent problems with the arteries and veins, and of course also problems in the urinary tract."
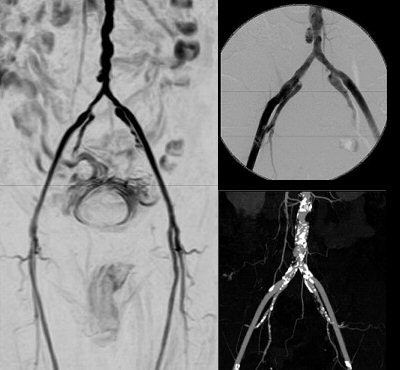
Rather than abandoning the contrast agent, Dr. Shawn Cowper suggested protocols that would minimize exposure to gadolinium but without omitting it. Cowper is an assistant professor of dermatology and pathology at the Yale University School of Medicine in New Haven, CT, and was the first to identify NSF in renal-deficiency patients back in 2000. Yale's NSF Registry, which Cowper oversees, contains data from 250 patients to date, most of whom received gadolinium prior to acquiring NSF.
 |
| Above, noncontrast subclavian MRA on 1.5-tesla scanner (Achieva). Below, noncontrast renal 3D TFE MRA with inversion recovery of stenosis of the renal arteries using navigator gating during free breathing. Images courtesy of Philips Medical Systems North America, Hyogo Brain and Heart Center, Hyogo, Japan (above), and Dr. Marcus Katoh, University of Aachen, Germany (below). |
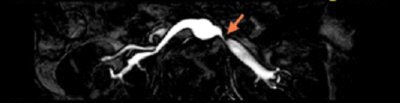 |
"Folks here at Yale have rethought various patient scenarios and developed new protocols aimed at minimizing exposure to gadolinium," Cowper told AuntMinnie.com. "There is an impression that macrocyclic agents (such as gadoteridol, gadobutrol, and gadoterate) may be 'safer' than linear agents (such as gadodiamide and gadopentetate). Whether any sort of gadolinium use in renal patients is safe is very much a philosophical question."
Dr. Jeffrey Weinreb, chief of MRI at Yale School of Medicine, told AuntMinnie.com that his department would "certainly welcome any contrast agent -- with or without gadolinium -- that did not have this (NSF) complication." But as Cowper mentioned, Weinreb and colleagues are being proactive in their own right.
"Our approach at Yale is to develop alternative imaging algorithms for patients with poor renal function who would have, prior to the recent revelations about (gadolinium) and NSF, undergone contrast-enhanced MRI/MRA without a second thought," he explained. "We have a lot of tools in our armamentarium, including noncontrast-enhanced MR, ultrasound, CO2, and low-dose iodinated contrast angiography. These may not be the ideal techniques for making a diagnosis, but in many instances they are sufficient."
In an interview with AuntMinnie.com, Dr. Patrick Colletti, chief of MRI at LAC+USC Imaging Science Center in Los Angeles, said that the limitations of noncontrast MRI include "difficulty in characterizing liver, renal, and breast lesions, and long exam time with lower diagnostic confidence for extremity and renal MRA."
Pathologies that can be hard to detect confidently without contrast MRI include small brain metastases, small pituitary adenomas, and active multiple sclerosis plaques, as well as intradural and intramedullary spinal lesions, he explained. Myocardial perfusion and viability are impossible to diagnose without contrast, Colletti added.
"One of the strengths of body MRI is its ability to characterize different lesions," echoed Dr. Susan Ascher, co-director of the division of abdominal imaging at Georgetown University Hospital in Washington, DC. "For most body MRI applications, lesion characterization necessitates IV contrast."
Dr. Vivian Lee, chief scientific officer and professor of radiology, physiology and neuroscience at New York University Medical Center in New York City, agreed that some MRI protocols are only successful with gadolinium.
"For example, for the detection of hepatocellular carcinoma (HCC), T1- and T2-weighted imaging alone, even combined with diffusion-weighted imaging, may not be sensitive enough to detect HCC," Lee said. "Even with gadolinium contrast, we know our sensitivity for small lesions is still low. Here, the use of gadolinium clearly improves sensitivity and is important for diagnostic accuracy."
Docs weigh in again: Giving up gadolinium
As much as gadolinium brings to an MR exam, imaging experts are not completely aghast at the notion of noncontrast MR. In fact, radiologists interviewed by AuntMinnie.com work quite satisfactorily with noncontrast MR in many instances.
"The majority of MRA is still nonenhanced and works perfectly well without contrast," said Dr. Emanuel Kanal, director of Magnetic Resonance Services and professor of radiology and neuroradiology at the University of Pittsburgh Medical Center in Pennsylvania. "The biggest area where contrast is given in high concentrations is MRA exams looking for metastatic disease in the brain, and body non-neuro MR angiograms. So the question is: Can we avoid requiring a contrast dynamic bolus MR angiogram on these patients?"
To that end, "2D time-of-flight MRA is a nonenhanced sequence that's an acceptable study for certain parts of the body," Kanal said. "For neuroradiologic applications like carotid imaging, it's actually not that bad of an idea if the patient is able to stay relatively motionless. Phase-contrast MRA, too, has pretty significant potential benefits for neuroradiologic imaging."
Colletti said that his institution has been using Magnevist (gadopentetate DTPA, Berlex, Montville, NJ) without incident, but agreed with Kanal that the majority of MR diagnoses could be made with currently available noncontrast techniques.
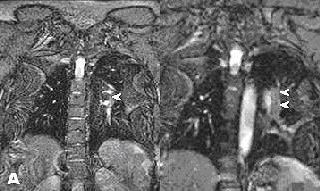 |
| Noncontrast-enhanced MR of pulmonary embolism. A 36-year-old woman, 12 weeks pregnant, presented with shortness of breath. Coronal (above) and axial (below) and axial FIESTA views demonstrate left main pulmonary artery embolus (arrowheads). Images courtesy of Dr. Patrick Colletti. |
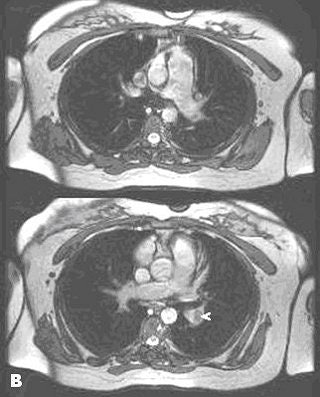 |
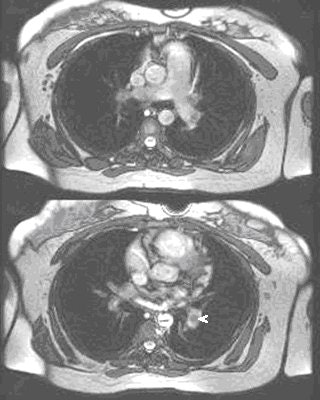 |
Ascher explained that, given the right circumstances, noncontrast MR can be a reliable diagnostic tool. "If an incidental adrenal mass is found on a CT study and the clinical question is whether the mass is an adrenal adenoma, we would do a noncontrast MRI with in- and out-of-phase spoiled gradient-echo sequences. In most instances, these noncontrast MR sequences would suffice," she said.
For Lee, nontumor applications, such as MR cholangiopancreatography (MRCP), can be performed quite readily without contrast material in most cases, she said.
"For body imaging, we do adjust our protocols depending on the indications of patients and the risks of gadolinium or CT imaging, and we're developing non-CE protocols for certain cases," Lee added.
Lee is involved in developing Siemens' HASTE technology. "Some newer non-CE MRA techniques, such as an ECG-gated fast spin echo, are very promising for faster MRA studies of the extremities," she said. "We have also been performing TrueFISP-based MRA. New sequences, which may use ASL, are very promising for applications in the chest and abdomen."
Go gad or no gad?
To expect gadolinium-enhanced MR to disappear completely would be a flight of fancy. As the experts pointed out, in many instances, contrast is vital for reliable imaging results. And the majority of MR specialists are "fully reliant on contrast-enhanced methods," and may shy away from noncontrast methods, Lee said.
But the regulatory climate, and that pesky malpractice pothole, may force these same radiologists to rethink their position on NSF. In May, the U.S. Food and Drug Administration (FDA) asked that manufacturers of gadolinium-based MRI contrast agents include a "black box" warning on their product labeling.
European officials, such as the U.K. Commission on Human Medicines, have also taken action. A European committee on medicine safety has advised against using gadodiamide in patients with severe kidney failure, and advised that a warning should be added to product information regarding the use of gadodiamide in neonates because of their immature kidney function. The commmittee also advised that a strong warning about NSF be added to the product information for other gadolinium-containing agents marketed in the European Union.
Back in March, the Canadian health regulatory agency issued an advisory, urging healthcare professionals to "carefully consider the risks and benefits of performing MR with (gadolinium) in patients with moderate to severe kidney disease" (Health Canada, March 7, 2007).
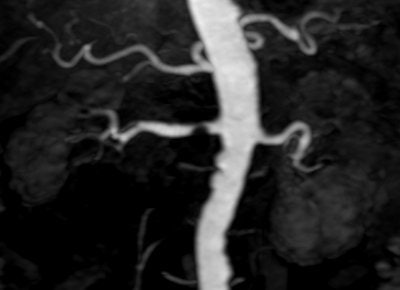
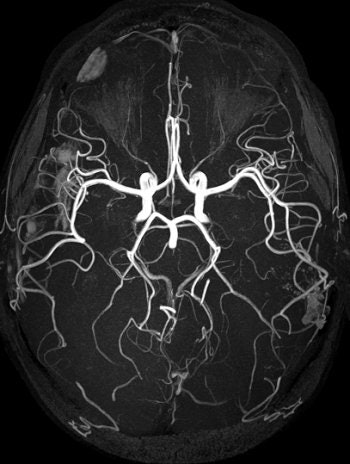 |
| TOF MRA on 3-tesla scanner offers significant vessel detail. Protocol: SENSE x2, 150 slices, 1,024 matrix, 0.25 x 0.45 x 0.6 mm, scan time of six minutes. Image courtesy of Philips Medical Systems North America and University of Bonn, Germany. |
Proceeding with caution is the safest bet for now. Each gadolinium formulation is manufactured differently in terms of how it binds to its chelate. Colletti recommended avoiding "gadodiamide or gadoversetamide, as these agents are up to 1,000 times less thermodynamically stable and have much higher chelate concentrations compared with the three more stable agents: gadoteridol, gadopentetate dimeglumine, and gadobenate dimeglumine. Avoid increased dosage examinations, if possible."
Peters asked that radiologists exercise their best judgment on a case-by-case basis, citing a parallel everyone can identify with: "Imagine we found out that aspirin, even in low doses, causes gastrointestinal bleeding -- which it does in a substantial number of patients -- resulting in significant morbidity and mortality," he said. "Thus, we take aspirin off the market or ban it."
"This appears straightforward on the first view, but is wrong in a global risk/benefit perspective," Peters pointed out. "Aspirin is of clear benefit for patients with coronary heart disease, on top of its risks. Banning it would exclude heart patients from the overall benefits of this drug, and thereby silently cause morbidity and mortality of these patients."
Finally, maintain an open mind to what noncontrast protocols have to offer, Lee advised. "In the cases where the non-CE protocols provide comparable accuracy to some of the CE protocols, such as for MRA, I believe these protocols are likely to catch on as long as the manufacturers are able to provide robust sequences that are user-friendly."
By Sydney Schuster
AuntMinnie.com contributing writer
July 26, 2007
Related Reading
FDA asks for 'black box' warning for gadolinium contrast, May 23, 2007
Gadolinium: A 'necessary factor' in the development of NSF?, March 27, 2007
Tyco hit with lawsuit over gadolinium reaction, March 19, 2007
Health Canada issues gadolinium advisory, March 15, 2007
Scottish study shows potential gadolinium-NSF link, March 9, 2007
Copyright © 2007 AuntMinnie.com