Paramed Medical Systems has received approval for a new MRI scanner, the company reported.
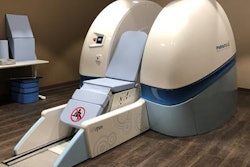
The U.S. Food and Drug Administration (FDA) has cleared MRopen, a 0.5-tesla cryogen-free superconductive MRI system, according to Paramed of Genoa, Italy. It uses a cable made of magnesium diboride (MgB2), an alloy with superconductive characteristics at temperatures that can be reached with a traditional cooling system.
MRopen doesn't require liquid helium or other cryogenic gases and is configured in a U-shaped design to avoid patient claustrophobia, the company said.
Related Reading
Road to RSNA, Paramed Medical Systems, October 29, 2007
Italian firm turns MRI on its head with new magnet design, January 15, 2007
Paramed introduces MRopen, November 6, 2006
Copyright © 2008 AuntMinnie.com