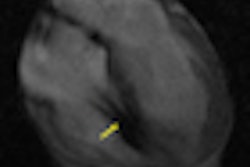
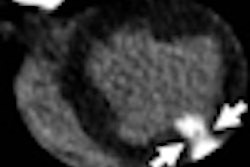
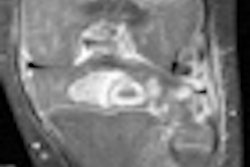
The magnetic pulses from an MRI scan may mix with the electronic pulses from a pacemaker, potentially endangering a patient undergoing MR-based imaging procedures.
Research published online by BioMed Central's BioMedical Engineering OnLine cites a study by U.S. Food and Drug Administration (FDA) researchers, who cautioned that certain cardiac pacemakers may inadequately stimulate a patient's heart while undergoing an MRI scan.
FDA researchers Howard Bassen and Gonzalo Mendoza evaluated the risk of pacemakers causing unintended cardiac stimulation following exposure to a simulated MRI magnetic field by measuring electrical voltage produced at the tip of the pacemaker lead, where it would touch the interior of the heart.
The researchers found that when exposed to the strong magnetic field, a pacemaker could deliver a drastically altered pulse and stimulate the heart inappropriately. Such an inadvertent pulse could cause severe issues for a patient.
Bassen and Mendoza noted that MRI systems "emit several types of extremely intense magnetic fields and have caused injury to patients due to interactions with pacemakers. Cardiologists who choose to scan patients with cardiac pacemakers must assess the risks versus the benefits of the scan."
Related Reading
Medtronic touts MRI pacemaker study, May 14, 2009
MRI OK for patients with newer implanted cardiac devices, November 25, 2007
MRI of cardiac devices OK, but requires following strict guidelines, January 30, 2007
Radiologists should discuss cardiac implant disposal with patients, January 1, 2007
High-field MRI of the brain safely acquired in pacemaker patients, December 15, 2006
Copyright © 2009 AuntMinnie.com