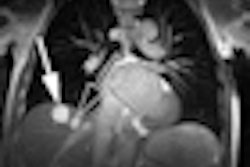
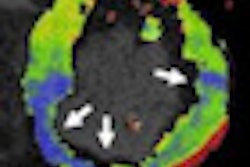
Philips Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Ingenia MRI system.
The system, available in 1.5-tesla and 3-tesla versions, performs digital signal acquisition and processing directly at the location of the patient.
By digitizing the signal directly into the radiofrequency receive coil nearest the patient and transferring and processing the signal in digital form throughout the imaging chain, Ingenia is able to generate up to a 40% improvement in signal-to-noise ratio compared to previous-generation systems, according to Philips.
The new MRI scanner has a 70-cm bore, providing whole upper-body imaging in just two stations, and coverage for total body without manipulation of the patient or coil.