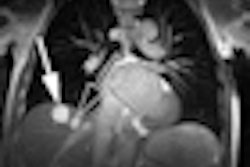
The U.S. Food and Drug Administration (FDA) has cleared GE Healthcare's GEM Suite (geometry embracing method) of surface coils designed for use with the company's Optima MR450w 1.5-tesla wide-bore MRI system.
The suite features a set of receive-only radiofrequency surface coils, including an express table and GEM posterior array, a head and neck unit, an anterior array, a peripheral vascular/lower extremity array, and flex coils.
The coils can be used individually or combined to provide comprehensive patient coverage, according to GE. The suite allows for automatic selection of the configuration that best fits the selected region of interest, and it includes a total 205-cm scanning range and feet-first scanning, GE said.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











