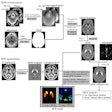
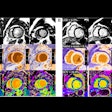
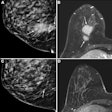
Image-guided therapy developer IMRIS has received a Health Canada medical device license for AccuTrack, the company's 3D image guidance tool for use in IMRIS neurosurgery suites.
AccuTrack's new software-enabled capability helps surgeons make decisions by utilizing a lightweight probe to localize target anatomy at any time during a neurosurgical procedure, the company said.
AccuTrack has already received CE Mark regulatory approval in Europe and is pending 510(k) approval from the U.S. Food and Drug Administration (FDA), according to IMRIS.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)