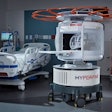
Focused-ultrasound developer InSightec has submitted a premarket approval (PMA) application to the U.S. Food and Drug Administration (FDA) for its ExAblate treatment for palliation of pain from bone metastases.
The FDA has granted an expedited review process for the PMA submission.
ExAblate uses MR-guided focused ultrasound (MRgFUS) to treat pain caused by bone metastases noninvasively and without ionizing radiation.
ExAblate is the only MRgFUS system with FDA approval, granted in 2004 for uterine fibroids. It also has the European CE Mark for treatment of uterine fibroids, bone metastases, and adenomyosis.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)

.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)