Siemens Healthcare has received U.S. Food and Drug Administration (FDA) clearance for its Magnetom Spectra 3-tesla MR scanner.
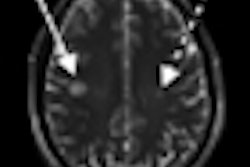
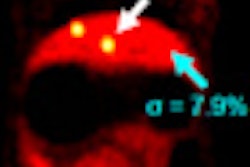
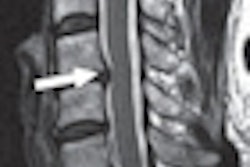
Targeted at a lower price point, Spectra includes Siemens' Tim 4G coil technology and Dot workflow capabilities, according to the vendor. It can be used for a broad spectrum of applications, from diagnosing damaged ankle cartilage to conducting dynamic abdominal examinations and performing functional brain imaging, Siemens said.
Siemens pointed to Spectra's cableless direct-connect coils, which allow for up to 120 coil elements to be set up quickly. Large scans, such as whole-body scans up to 80.71 inches, can be performed in one examination without patient or coil repositioning, according to Siemens.